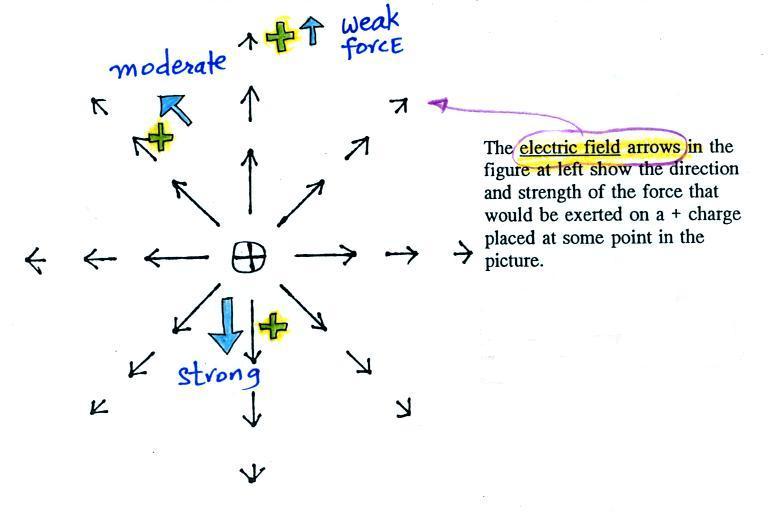
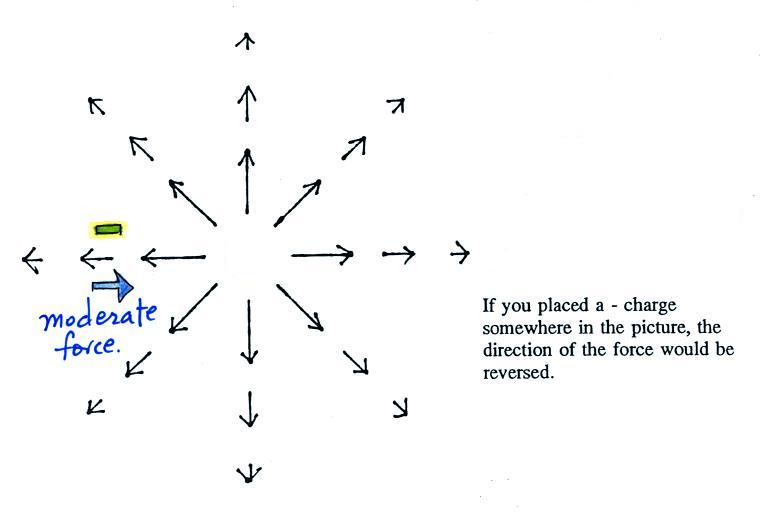
What is the direction of the
electric field arrow at Point X halfway between a + and a -
charge?
The second question has two parts. First you need to determine
what polarity of charge must be on ground to cause the charges in the
figure below to move as they are doing. Then what direction does
the electric field arrow point at a location just above the ground
where the two charges are found.