Try to read through this material before class on Mon., Mar. 23.
click here to download these
notes in a more printer friendly format
The
following is an
introduction to an important new topic: humidity (moisture in the
air). This topic and the terms that we will be
learning and using can be confusing. That's the reason for this
introduction. We will be mainly be
interested in 4 variables, what they are
and what can cause their values to change. The variables are :
mixing ratio, saturation
mixing ratio, relative humidity, and dew point. You will find
much of what follows on page 83 in the photocopied ClassNotes.
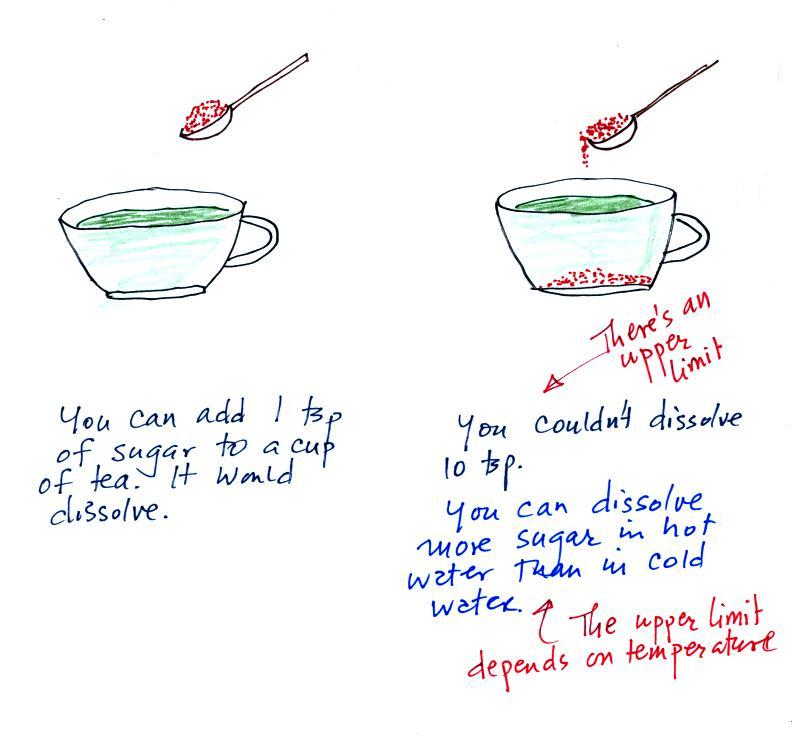
Mixing ratio tells you how much water vapor is actually in
the
air. Mixing ratio has units of grams of water vapor per kilogram
of dry air (the amount of water vapor in grams mixed with a
kilogram
of dry air). It is basically the same
idea as teaspoons of
sugar
mixed in a cup of tea. You may be tempted to click on the words
highlighted in blue. Most of these aren't links. One of
them is, it
will take you to a hidden optional assignment.

The value of the mixing ratio won't change unless you add
water
vapor to or remove water vapor from the air. Warming the air
won't
change the mixing ratio. Cooling the air won't change the mixing
ratio
(unless
the air is
cooled below its dew point temperature and water
vapor starts to condense). Since the mixing ratio's job is to
tell you
how much water vapor is in the air, you don't want it to change unless
water vapor is added to or removed from the air.
Saturation mixing ratio is just an upper limit to how much
water vapor
can be found in air, the air's capacity for water
vapor. It's a
property of air, it doesn't say anything about how much water
vapor is actually in the air (that's the mixing ratio's job).
Warm air can potentially hold more
water vapor than cold air.
This variable has the same units: grams of water vapor per kilogram of
dry air. Saturation mixing ratio values for different air
temperatures are listed and graphed on p. 86 in the photocopied class
notes.
Just as is the case with water vapor in air,
there's a limit to how much sugar can be dissolved in a cup of hot
water. You can dissolve more
sugar in hot water than in cold
water.
The dependence of saturation mixing ratio on air temperature is
illustrated below:
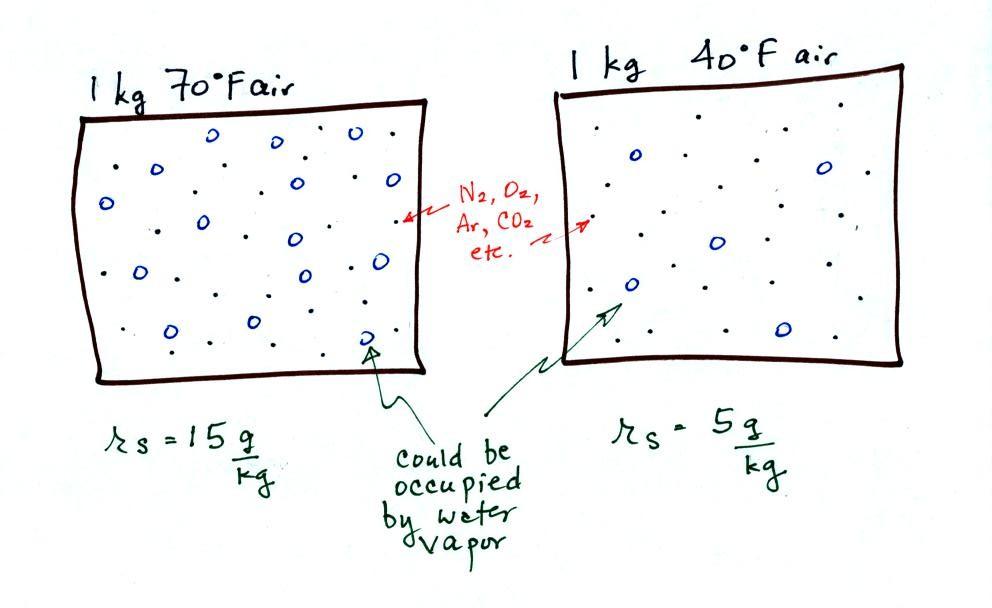
The small specks represent all of the gases in
air except
for the water
vapor. Each of the open circles represents 1 gram of water vapor
that
the air could
potentially hold. There are 15 open circles
drawn in the 1
kg of 70 F air; each 1 kg of 70 F air could hold up to 15 grams of
water vapor. The 40 F air only has 5 open circles; this
cooler
air can only hold up to 5 grams of water vapor per kilogram of dry air.
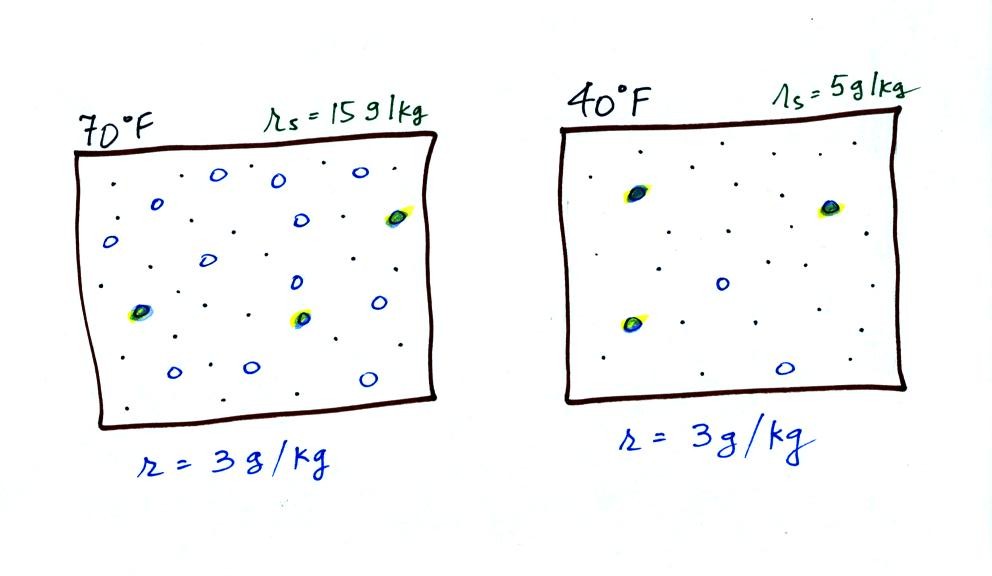
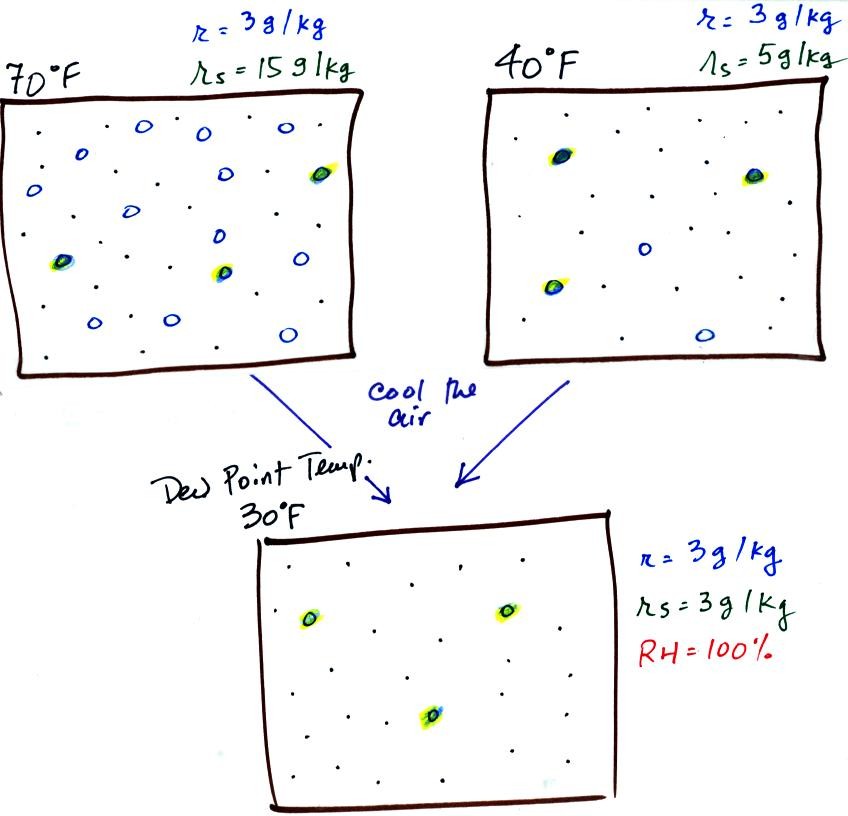
Now we have gone and actually put some water vapor
into the
volumes of
70 F and 40 F air. The same amount, 3 grams of water vapor, has
been added to each
volume of air. The mixing ratio, r, is 3 g/kg in both cases.
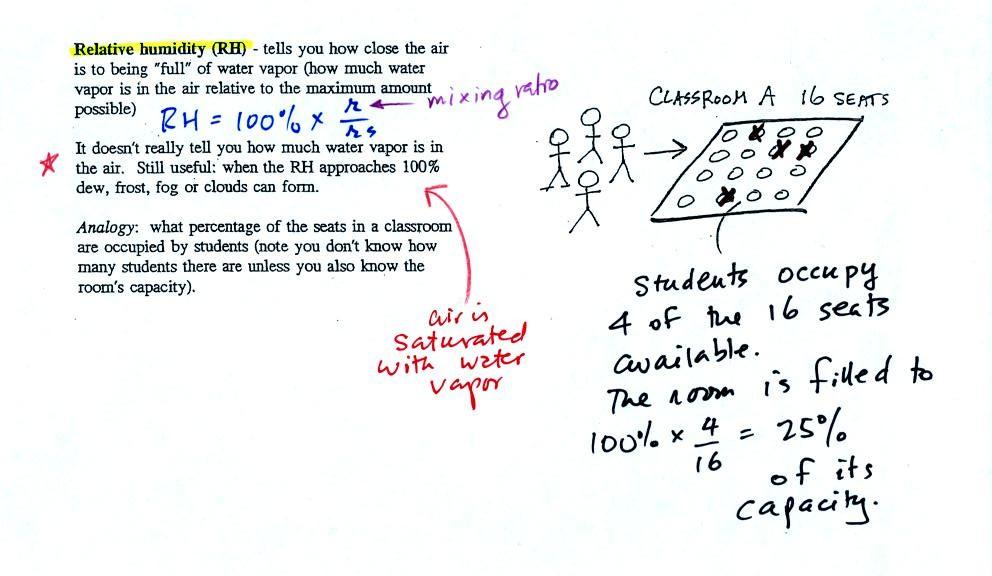
The relative
humidity is the variable most people are familiar with, it tells you
how "full" the air is with water
vapor.
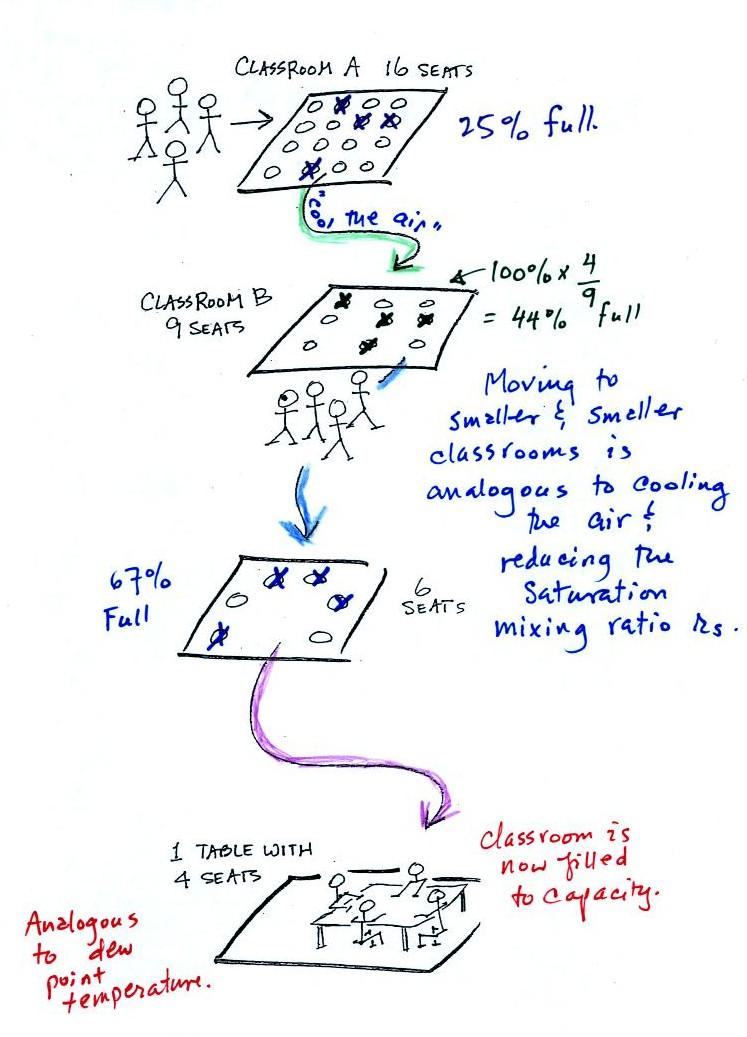
In the analogy (sketched on the right hand side of p. 83 in
the photocopied notes) 4 students wander into Classroom A which has 16
empty
seats. Classroom A is filled to 25% of its capacity.
You can think of 4, the number of students, as being analogous to the
mixing ratio. The classroom capacity is analogous
to the
saturation mixing ratio. The percentage occupancy is analogous to
the relative humidity.
Instead of students and a classroom you
could think of the 70 F and 40 F air that could potentially hold 15
grams or 5 grams, respectively of water vapor. Here is the
Optional Assignment I mentioned I would hide in these notes. It
will be due at the beginning of class on Mon., Mar. 23.
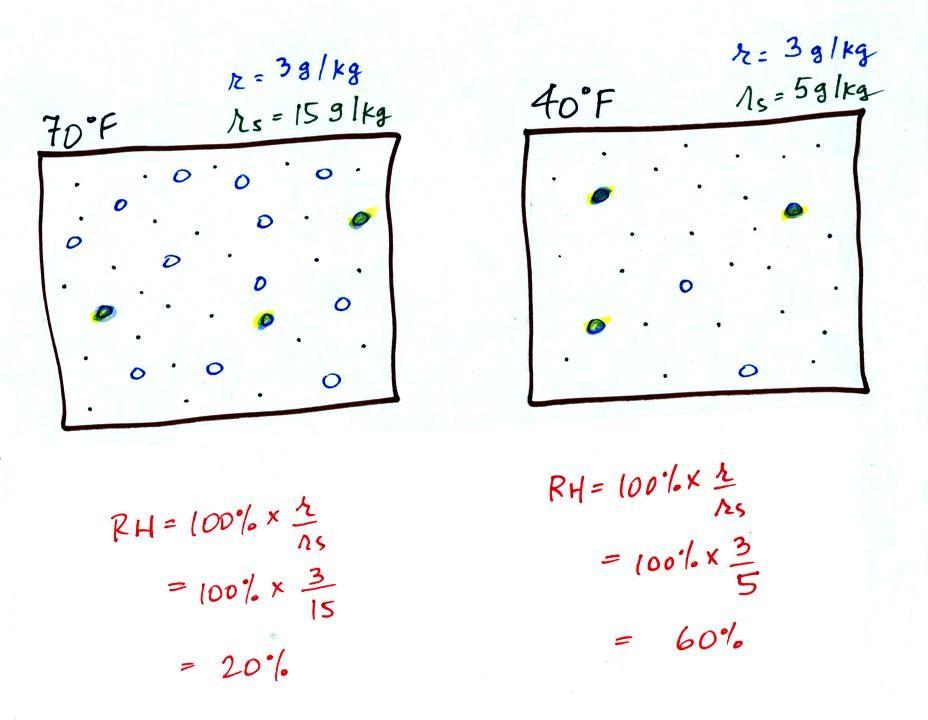
Here are the relative humidities of the 70 F and 40 F air
that each
contain 3 grams of water vapor. The 70 F air has a low RH because
this warm air's saturation mixing ratio is large. The RH in the
40 F is higher even though it has the same actual amount of water vapor
because the 40 F air can't hold as much
water vapor and is closer
to
being saturated.
Something important to note: RH doesn't really tell you how much water
vapor is
actually in the air. The two volumes of air above contain the
same amount of water vapor (3 grams per kilogram) but have different
relative humidities. You could just as easily have two volumes of
air with the same relative humidities but different actual amounts of
water vapor.
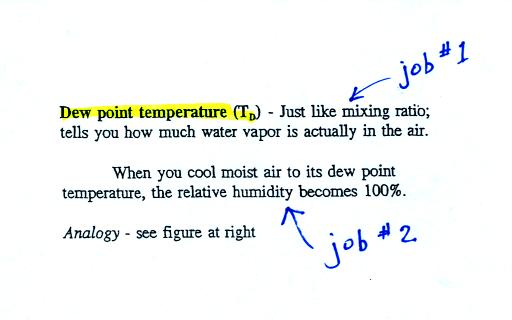
The dew point temperature has two jobs. First it gives you an
idea of
the actual amount of water vapor in the air. In this respect it
is just like the mixing ratio. If the dew point temperature is
low the air doesn't contain much water vapor. If it is high the
air contains more water vapor.
Second the dew point tells you how much you must cool the air in order
to cause the RH to increase to 100% (at which point a cloud, or dew or
frost, or fog would form).
If we cool the 70 F air or the 40 F air to 30 F we would
find that the
saturation mixing ratio would decrease to 3 grams/kilogram. Since
the air actually contains 3 g/kg, the RH of the 30 F air would become
100%. The 30 F air would be saturated, it would be filled to
capacity with water vapor. 30 F is the dew point temperature for
70 F air that contains 3 grams of water vapor per kilogram of dry
air. It is also the dew point temperature for 40 F air that
contains 3 grams of water vapor per kilogram of dry air.Because
both volumes of air had the same amount of water vapor,
they both
also have the
same dew point temperature.
Now back to our students and classrooms analogy on the
righthand
side of p. 83. The 4 students
move into classrooms of smaller and smaller capacity. The
decreasing capacity of the classrooms is analogous to the
decrease in saturation mixing ratio that occurs when you cool
air. Eventually the students move into a classroom that they just
fill to capacity. This
is analogous to cooling the air to the dew point.
If the 4 students were to move to an even smaller classroom, they
wouldn't all fit inside. The same is true of moist air. If
you cool
moist air below the dew point, some of the water vapor will
condense.