| Indicator | Low pH color | Transition pH range | High pH color |
|---|---|---|---|
| Gentian violet (Methyl violet 10B) | yellow | 0.0–2.0 | blue-violet |
| Leucomalachite green (first transition) | yellow | 0.0–2.0 | green |
| Leucomalachite green (second transition) | green | 11.6–14 | colorless |
| Thymol blue (first transition) | red | 1.2–2.8 | yellow |
| Thymol blue (second transition) | yellow | 8.0–9.6 | blue |
| Methyl yellow | red | 2.9–4.0 | yellow |
| Bromophenol blue | yellow | 3.0–4.6 | purple |
| Congo red | blue-violet | 3.0–5.0 | red |
| Methyl orange | red | 3.1–4.4 | orange |
| Bromocresol green | yellow | 3.8–5.4 | blue |
| Methyl red | red | 4.4–6.2 | yellow |
| Methyl red | red | 4.5–5.2 | green |
| Azolitmin | red | 4.5–8.3 | blue |
| Bromocresol purple | yellow | 5.2–6.8 | purple |
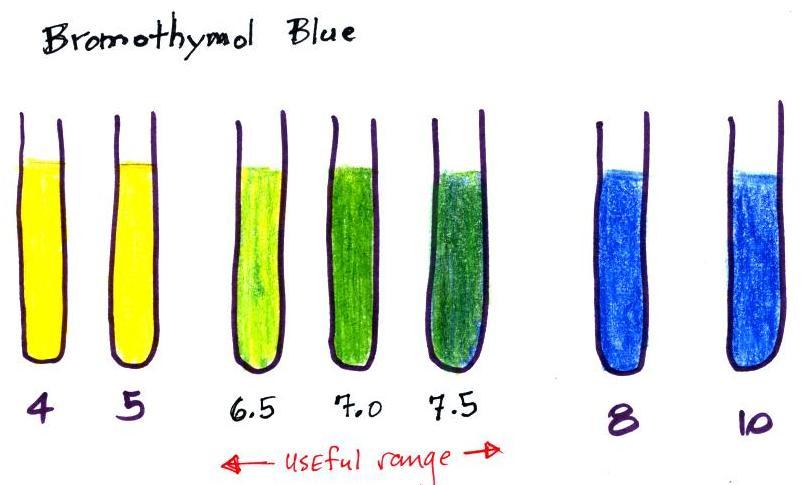
| Bromothymol blue | yellow | 6.0–7.6 | blue |
| Phenol red | yellow | 6.4–8.0 | red |
| Neutral red | red | 6.8–8.0 | yellow |
| Naphtholphthalein | colorless to reddish | 7.3–8.7 | greenish to blue |
| Cresol Red | yellow | 7.2–8.8 | reddish-purple |
| Phenolphthalein | colorless | 8.3–10.0 | fuchsia |
| Thymolphthalein | colorless | 9.3–10.5 | blue |
| Alizarine Yellow R | yellow | 10.2–12.0 | red |
