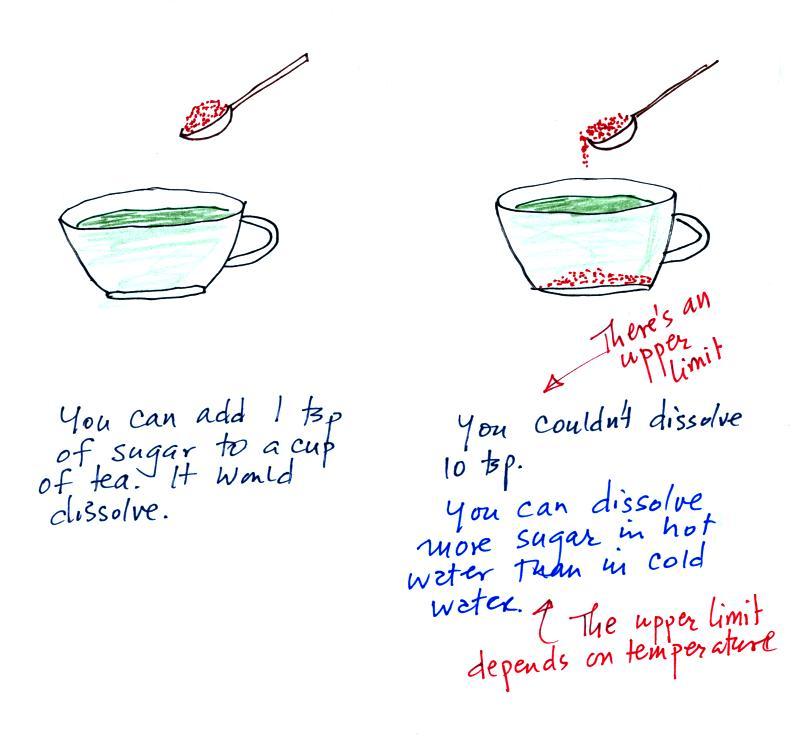
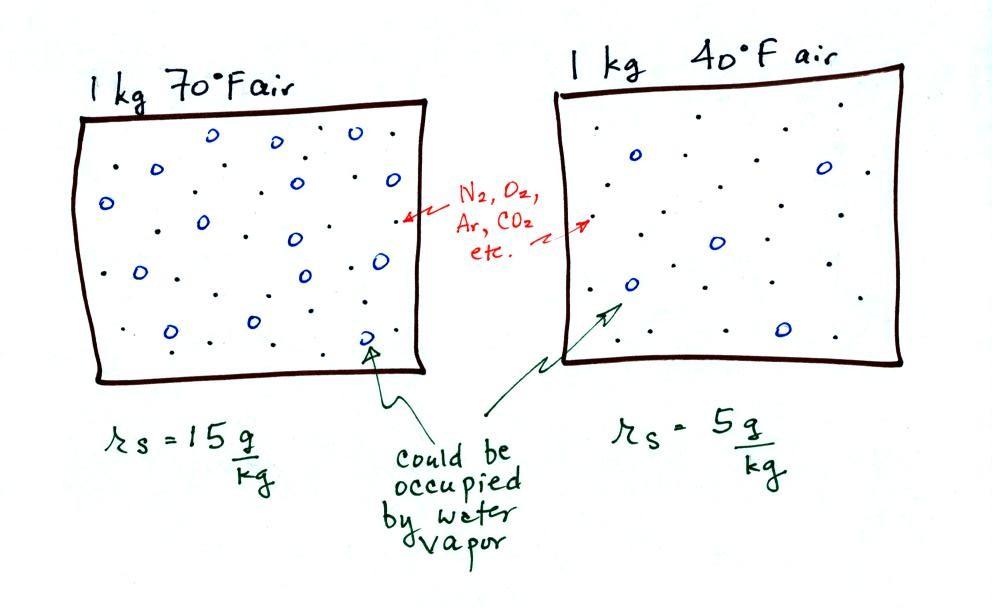
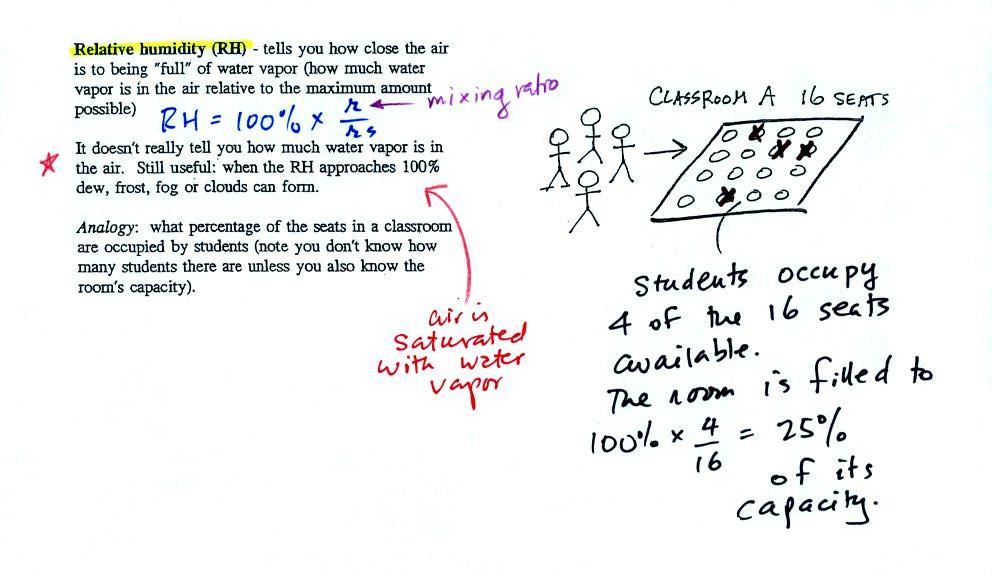
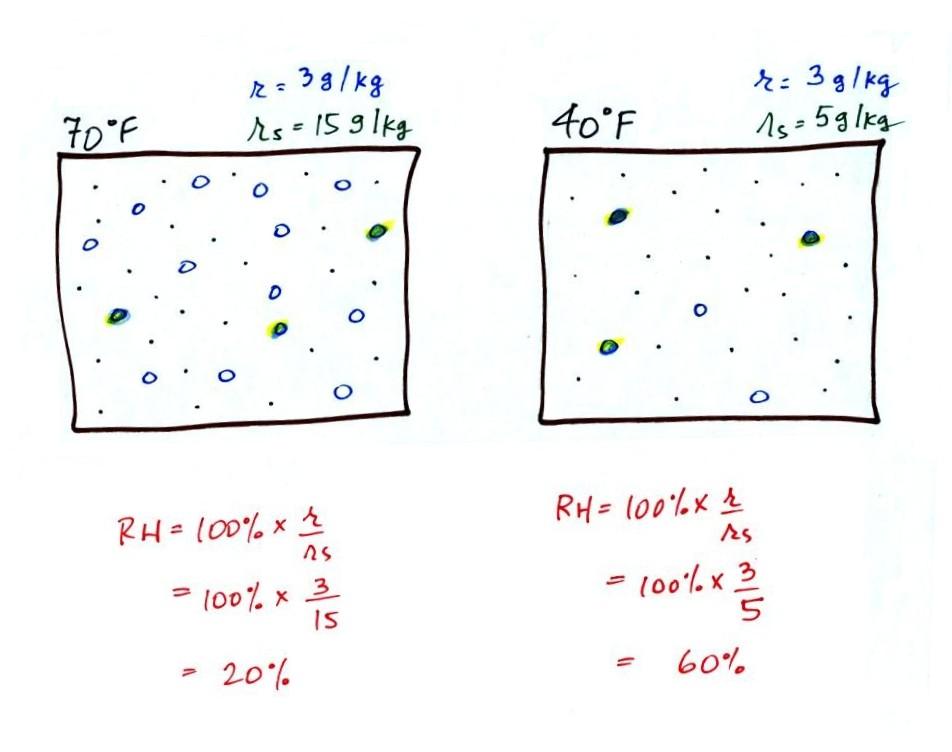
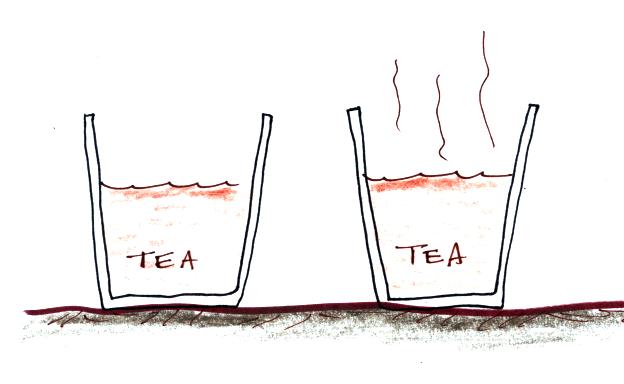
The first thing we need to realize is that warm water will
evaporate more rapidly than cool water. You probably know that
already. If a cup of iced tea were set next to a cup of hot tea
you probably be able to tell which was which by just looking at
them. You wouldn't need to touch or taste the tea or look for ice
cubes in the iced tea.
You might notice that one of the
cups of tea was steaming (the cup on the right above). This would
be the hot tea. You're not actually seeing water vapor.
Rather water vapor is evaporating so quickly that it is saturating the
air above. The air isn't able to accomodate that much water vapor
and some of it condenses and forms a cloud of steam. That's what
you are seeing.
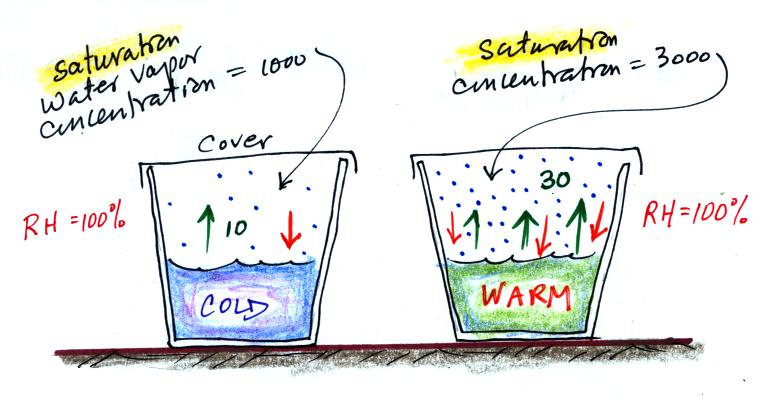
Now we'll redraw the picture and cover both cups so that water
vapor can begin to buildup in the air above the water in both
cups.
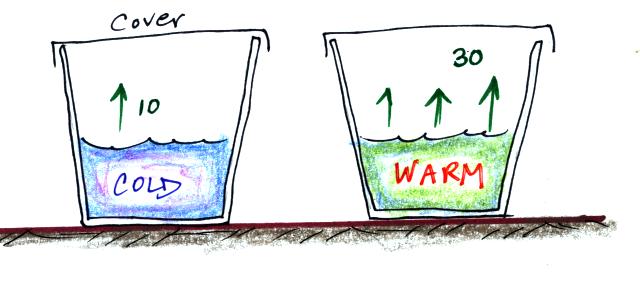
Water vapor will start to buildup in the air above each cup. And,
even though it has just evaporated, some of the water vapor will
condense and rejoin the water at the bottom of each cup. Let's
just assume that 1% of the water vapor molecules will condense.
The water vapor concentration in each glass will increase until it
reaches a point where
water evaporation rate = water
vapor condensation rate
for the cup of cold water
10 = 0.01 x water vapor
concentration
The 0.01 is 1% expressed in decimal form. Solving
this
equation
gives you a water vapor concentration of 1000. The
air is saturated when you reach this point and the RH = 100%.
The saturation water vapor concentration in the air in the warm
cup would be 3000. And again the relative humidity would be 100%.
The fact that the rates of evaporation and condensation are equal when
air is saturated (RH = 100%) is something we'll be using later when we
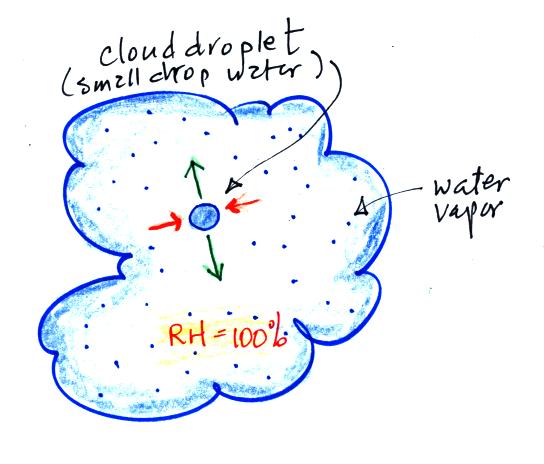
study the formation of precipitation. Here's a picture of how
that would look inside a cloud.
The air inside the cloud is saturated. The rate of
evaporation from the cloud droplet (2 green arrows) is balanced by an
equal rate of condensation (2 orange arrows). The RH =
100%. The cloud droplet won't grow any bigger or get any smaller.
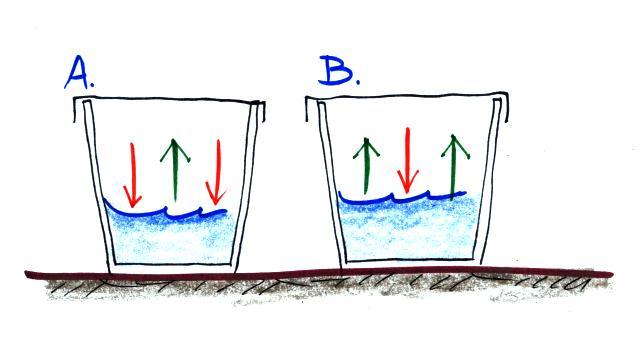
Here's something to test your understanding of this material.
What information can you add to this picture? Is the water in one
of the glasses warmer than the other? Is there more water vapor
in the air in one of the glasses than the other? Is the relative
humidity in each glass more than 100%, less than 100% or is it equal to
100%. The rates of evaporation and condensation aren't equal in
either glass, so the pictures will change with time. What will
the glasses look once they have reached equilibrium? Click here when you think you know the
answers.
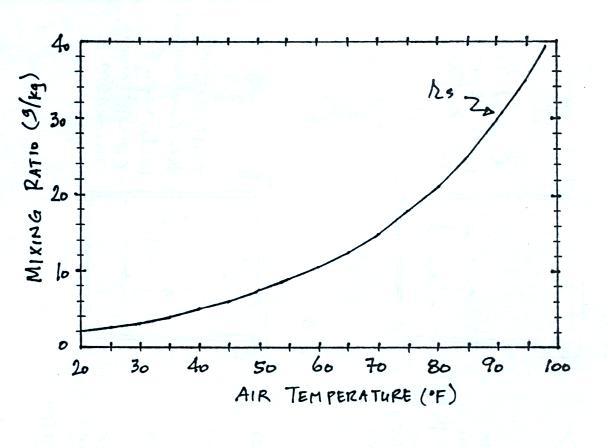
We'll
end this lecture with a table that shows the dependence of
saturation mixing ratio on air temperature.
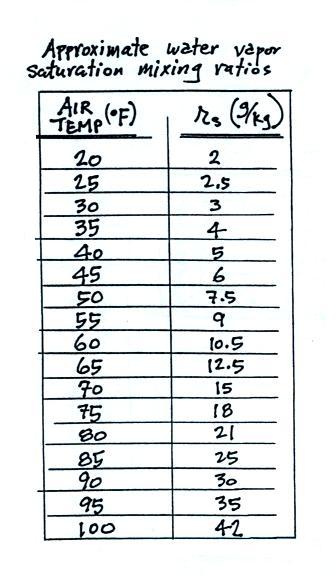
Note that the value of the
saturation mixing ratio doubles for every 20 F increase in
temperature.
The same data are shown in graphical form below.