Here are some rules governing the emission of electromagnetic
radiation:
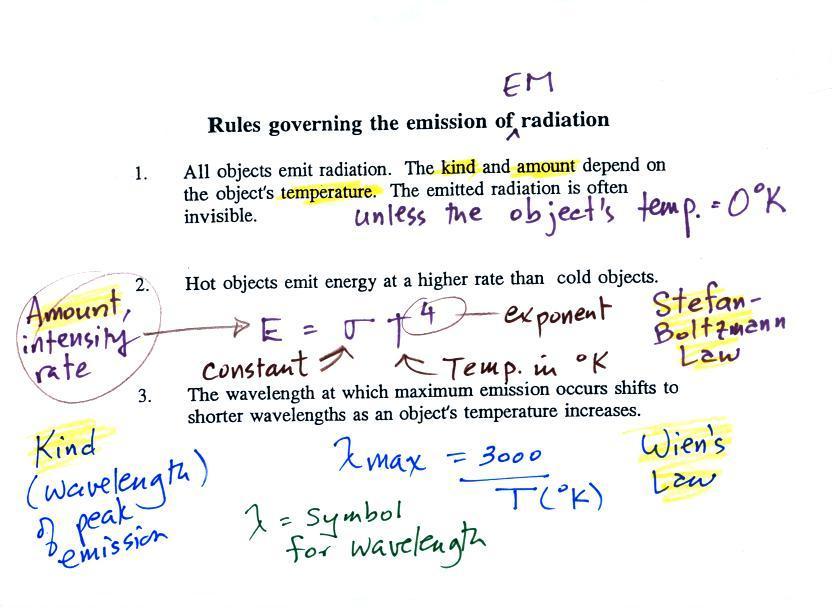
1.
Unless an object
is very cold (0
K) it will emit EM
radiation. Everything in a classroom: the people, the
furniture, the walls and the floor, even the air, are emitting EM
radiation. Often
this radiation
will be invisible so that we can't see it and weak enough that we can't
feel it. Both the amount and kind (wavelength) of the emitted
radiation depend on the object's temperature.
2.
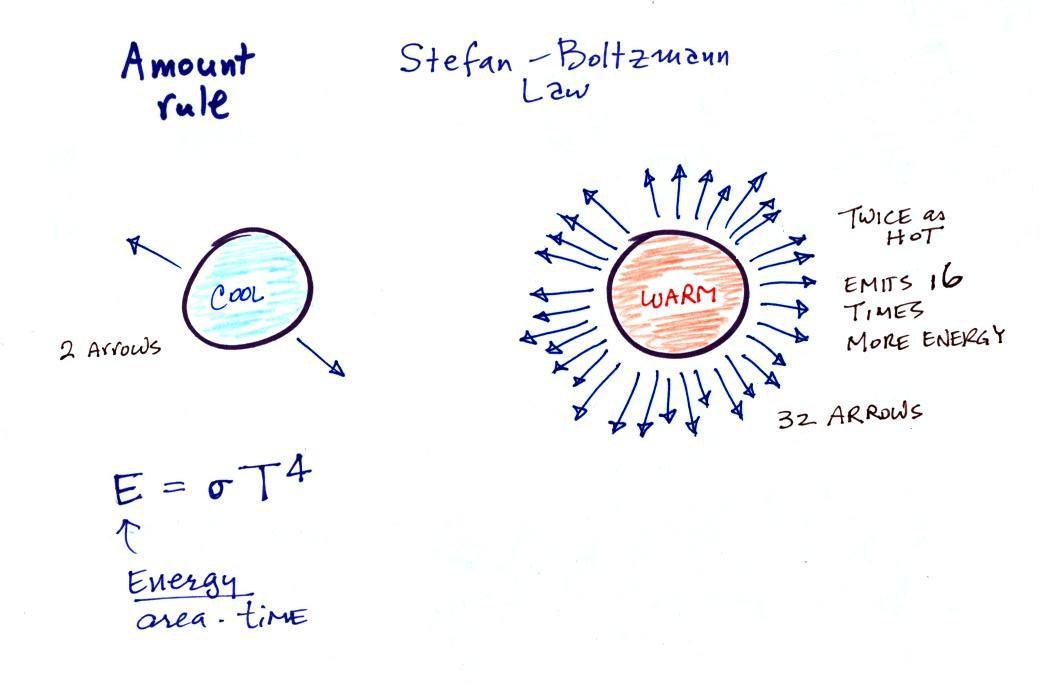
The second rule allows you to
determine the amount of EM radiation (radiant energy) an object will
emit. Don't worry about the units,
you can think of this as amount, or rate, or intensity (it is actually
energy per unit area per unit of time, calories per square centimeter
per minute for example).
Don't worry about σ either, it is just a
constant. The amount depends on temperature to
the fourth
power. If the temperature of an object doubles the amount of
energy emitted will increase by a factor of 2 to the 4th power
(that's 2 x 2 x 2 x 2 = 16). A hot object just doesn't emit a
little more energy than a
cold object it emits a lot more energy than a cold object. This
is illustrated in the following figure:
3.
The third rule tells you something
about the kind of radiation emitted
by an object. We will see that objects usually emit radiation at
many different wavelengths. There is one wavelength however at
which the object emits more energy than at any other wavelength.
This is called lambda max (lambda is the greek character used to
represent wavelength) and is called the wavelength of maximum
emission. The third rule allows
you to calculate "lambda max." This is illustrated below:
The
following graphs also help to
illustrate Stefan-Boltzmann's law
and Wien's law.
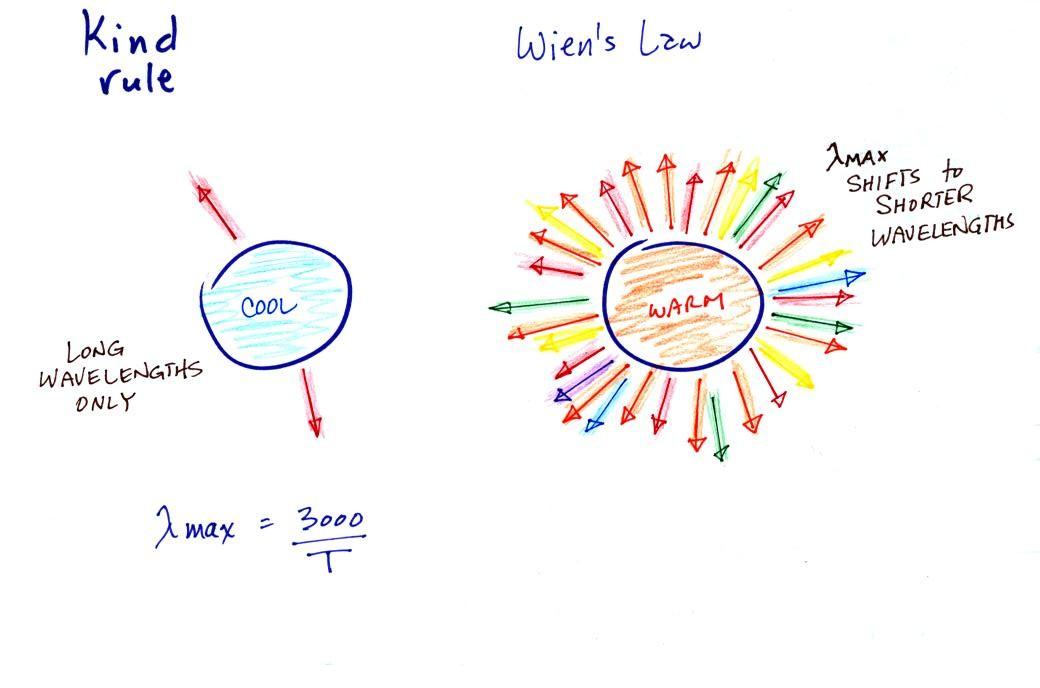
1.
Notice first that both the warm
and
the cold objects emit radiation
over a range of wavelengths (the curves above are like quiz scores, not
everyone gets the same score, there is a distribution of grades)
2.
Lambda max has shifted toward
shorter wavelengths for the warmer
object (red). This is Wien's law in action. The warmer
object is
emitting a lot of short wavelength radiation that the colder
object
(blue) doesn't emit.
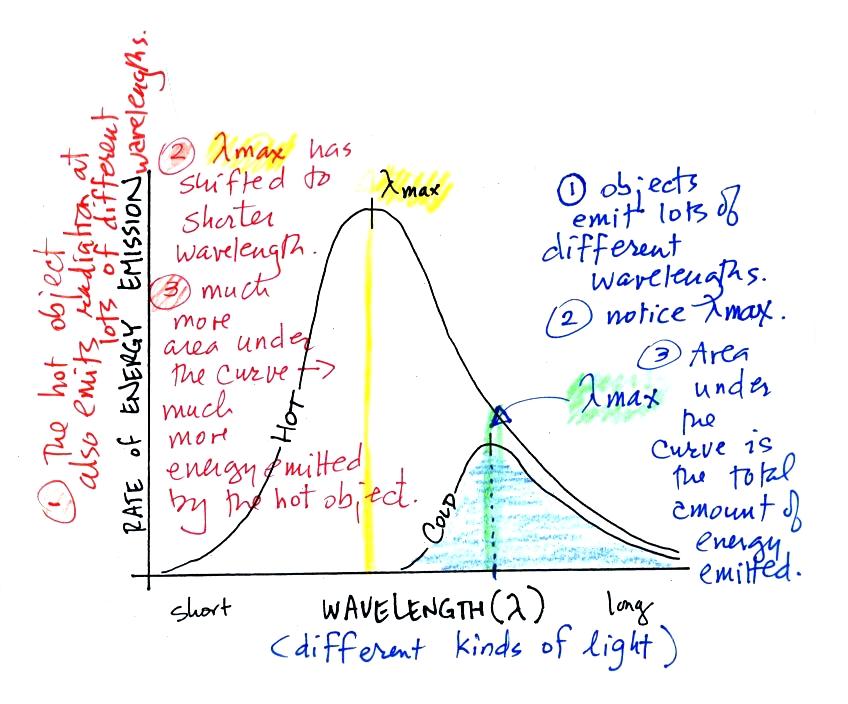
3.
The area under the warm object
curve is much bigger than the area under
the cold object curve. The area under the curve is a measure of
the total radiant energy emitted by the object. This illustrates
the fact that the warmer object emits a lot more radiant energy than
the colder object.
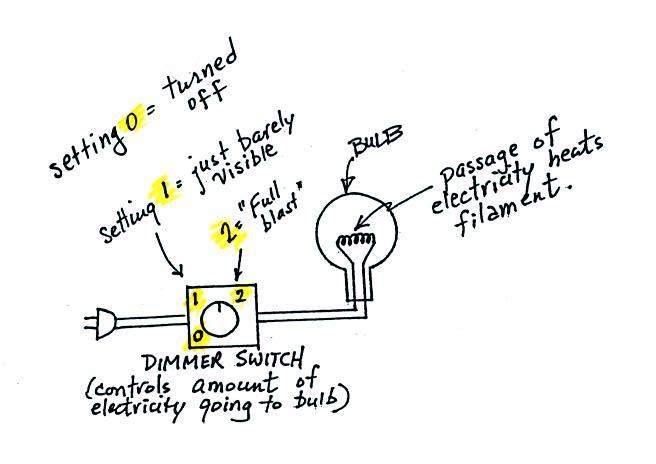
In the classroom version of this course, a 200 W tungsten bulb
connected to a dimmer switch is
used to demonstrate these rules We look at the EM
radiation emitted by the bulb
filament.
The three graphs below show the EM radiation emitted (both wavelength
and intensity) for the 3 switch settings in the figure above.
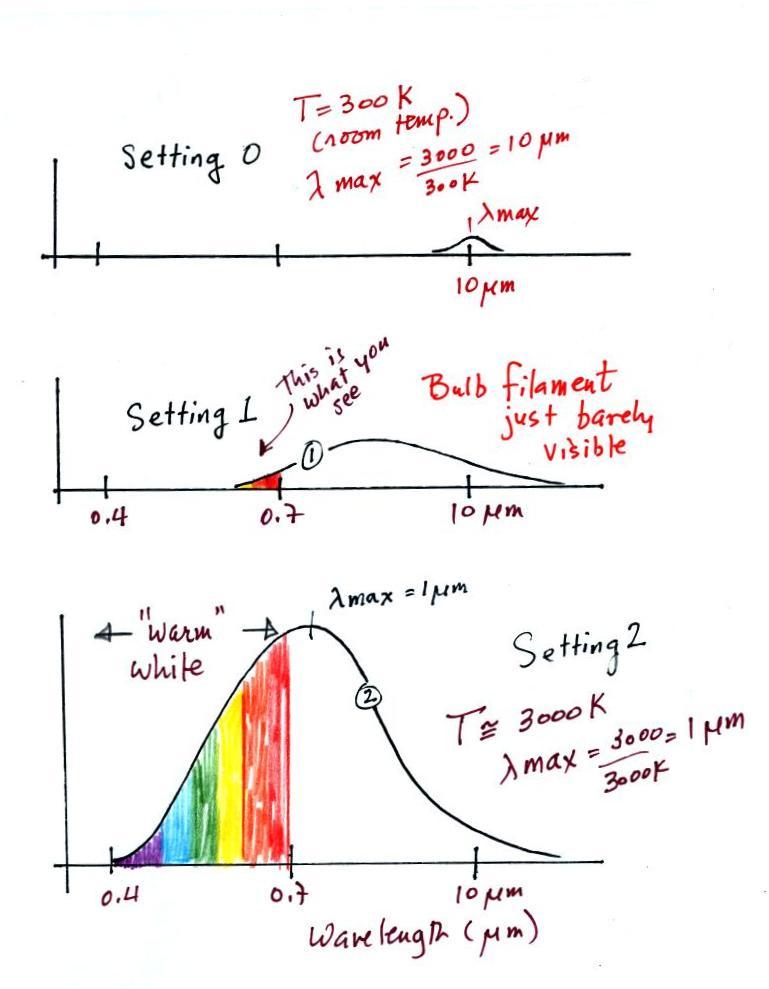
We start with the bulb turned off (switch Setting 0).
The
filament will be at room temperature which we will assume is around 300
K (remember that is a reasonable and easy to remember value for the
average temperature of the earth's surface). The bulb will be
emitting radiation, it's shown on the top graph above. The
radiation is very weak so we
can't
feel it. It is also long wavelength, far IR, radiation that we
can't see. The wavelength of peak emission is 10 micrometers.
Next we turn the dimmer switch to switch Setting 1 where the bulb's
filament just begins to glow (the
temperature of the filament is now about 900 K).
The bulb wasn't very bright at all and had an orange color. This
is curve 1, the middle figure. Note the far left end of the
emission curve has
moved left of the 0.7 micrometer mark and into the visible portion of
the
spectrum. That is glow from the filament that we are able to see,
just the small
fraction of
the radiation emitted by the bulb that is visible light (but just
long wavelength red and orange light). Most of the radiation
emitted by the bulb is to the right of the 0.7 micrometer mark and is
invisible IR radiation (it is strong enough now that you could feel it
if you put your hand next to the bulb).
Finally we turn on the bulb completely (it was a 200 Watt bulb so it
got
pretty bright). The filament temperature
is now about 3000 K. The bulb is emitting a lot more visible
light, all the colors, though not all in equal amounts. The
mixture of the colors produces a "warm
white" light. It is warm because it is a mixture that contains a
lot more red, orange, and yellow than blue, green, and violet
light. It is interesting that most of the radiation emitted by
the bulb is still in the IR portion of the spectrum (lambda max is 1
micrometer). This is
invisible light. A tungsten bulb like this is not especially
efficient source of visible light; more than half the light emitted by
the bulb is at IR wavelengths and is invisible.
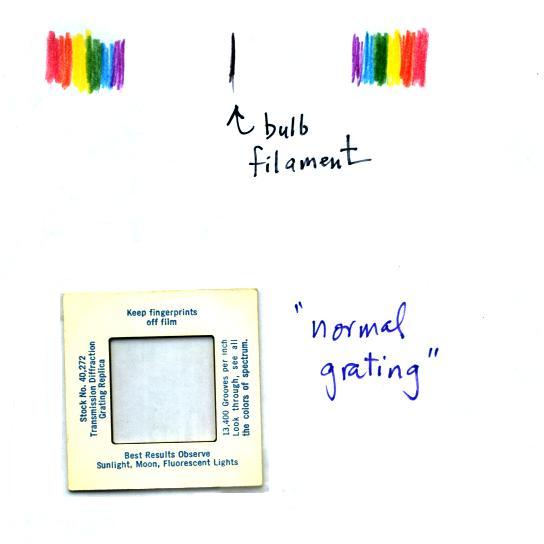
Diffraction gratings are handed out during this
demonstration. The students can use the diffraction gratings to
separate the
white light produced by the bulb into its separate colors. When
you look at the bright white bulb filament through one of the
diffraction gratings the colors are smeared out to the right and left
as shown below.
The sun
emits electromagnetic radiation. That shouldn't come as a surprise
since you can see it and feel it. The earth also emits
electromagnetic radiation. It is much weaker and invisible.
The kind and amount of EM radiation emitted by the earth and sun depend
on their respective temperatures.
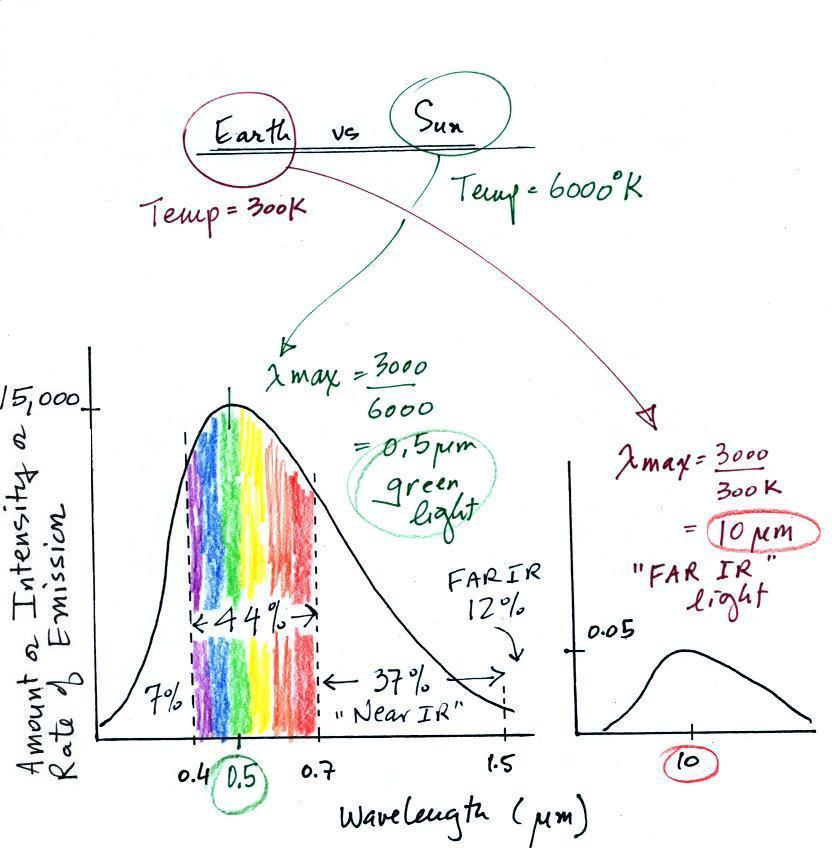
The curve on the left is for the sun. We first used Wien's
law and a temperature of 6000 K to calculate lambda max and got
0.5 micrometers. This is green light; the sun emits more green
light than any other kind of
light. The sun doesn't appear green because it is also emitting
lesser amounts of violet, blue, yellow, orange, and red - together this
mix of
colors appears white. 44% of the radiation emitted by the sun is
visible light, 49% is IR light (37% near IR + 12% far IR), and 7%
is ultraviolet light. More than half of the light emitted by the
sun is invisible.
100% of the light emitted by the earth (temperature = 300 K) is
invisible IR light. The
wavelength of peak emission for the earth is 10 micrometers.
Because the sun (surface of the
sun) is 20 times hotter than the earth a square foot of the sun's
surface emits energy at a rate that is 160,000 times higher than a
square foot on the
earth. Note
the vertical scale on the earth curve is different than on the sun
graph. If both the earth and sun were plotted with the same
vertical scale, the earth curve would be too small to be seen.
This is an appropriate point for a demonstration in the classroom
version of the course, a demonstration that might save students
some students some money (students that live off campus and pay
electric utility bills).
Ordinary tungsten bulbs (incandescent
bulbs) produce a lot of
wasted energy. They emit a lot of infrared light that is
wasted because it doesn't light up a room (it will heat up a room but
there are better ways of doing that). The light that they do
produce is a warm white color (tungsten bulbs emit lots of orange, red,
and yellow light and not as much blues, greens and violets). Energy
efficient
compact
fluorescent
lamps
(CFLs)
are
being
touted
as an ecological alternative to tungsten bulbs because
they use substantially less electricity, don't emit a lot of
wasted infrared light, and also last
longer. CFLs come with
different color temperature ratings.
The bulb with the hottest
temperature rating (5500 K ) in the figure
above is meant to mimic or simulate sunlight. The temperature of
the sun is 6000 K and lambda max is 0.5 micrometers. The spectrum
of the 5500 K bulb is similar.
The tungsten bulb (3000 K) and the CFLs with temperature ratings
of
3500 K and 2700 K produce a warmer white.
Three CFLs with the temperature ratings above are set up in class
so
that you could see the difference between warm and cool white
light. Personally I find the 2700 K bulb "too warm," it makes a
room
seem gloomy and depressing. The 5500 K bulb is "too cool" and
creates
a stark sterile atmosphere like you might see in a
hospital corridor. I prefer the 3500 K bulb in the
middle.
This figure below is from an article
on compact fluorescent lamps in Wikipedia. You can
see a clear difference between the cool white bulb on the left
in the figure below and the warm white light produced by a tungsten
bulb (2nd from the left) and 2 CFCs with low temperature ratings (3rd
and 4th from the left).
There is one downside to these energy efficient CFLs. The bulbs
shouldn't just be discarded in your ordinary household trash because
they contain mercury. They should be disposed of properly at a
hazardous materials collection site or perhaps at the store where they
were purchased.