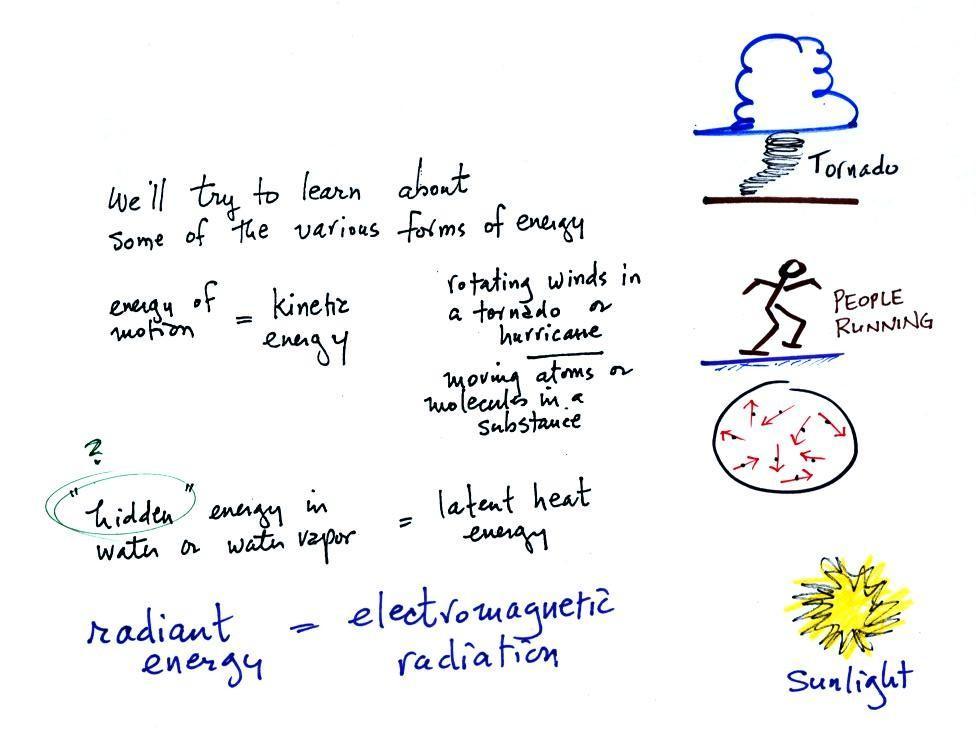
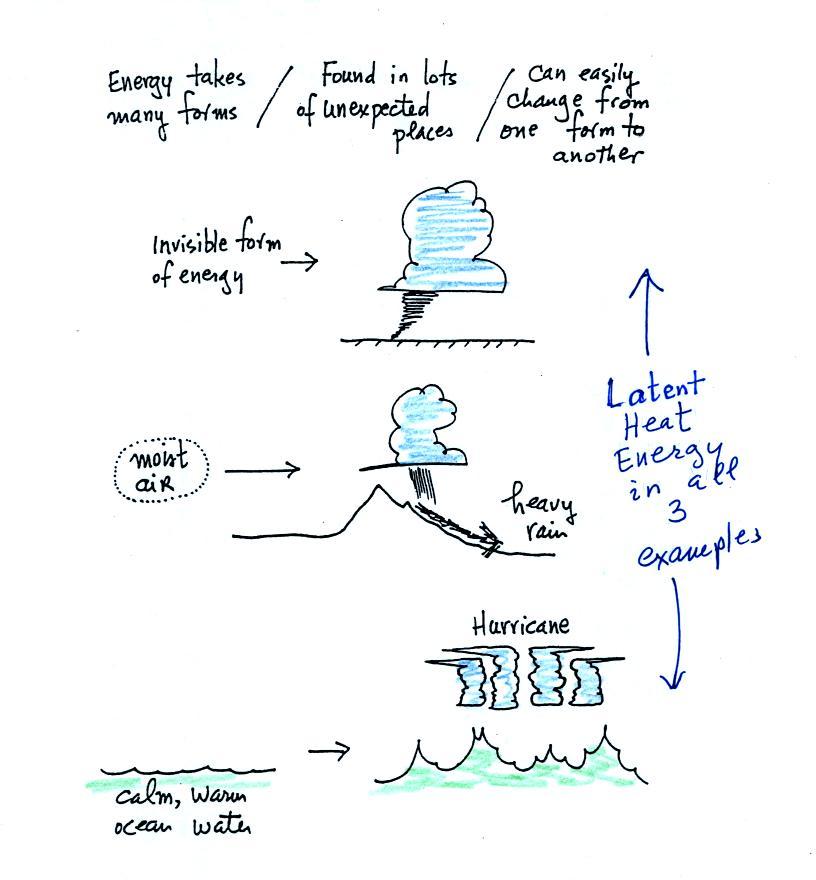
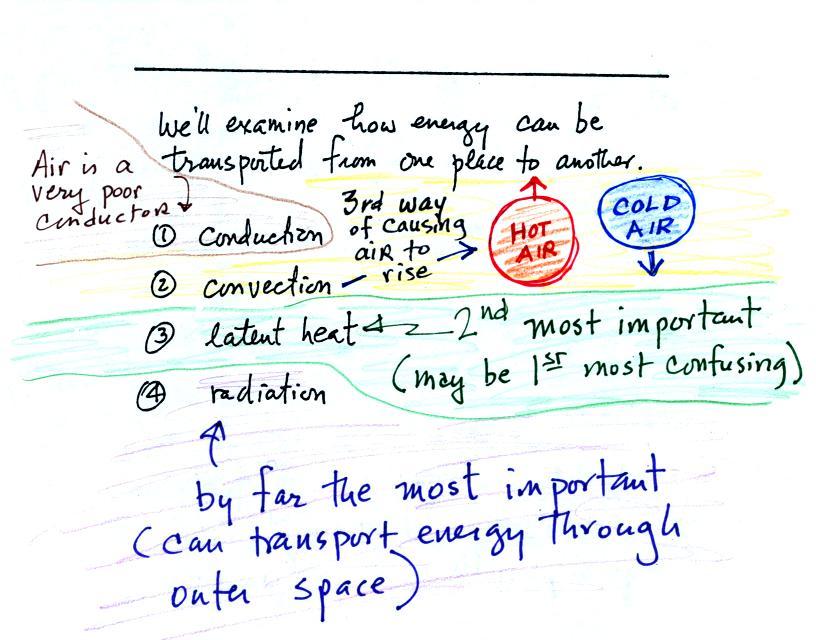
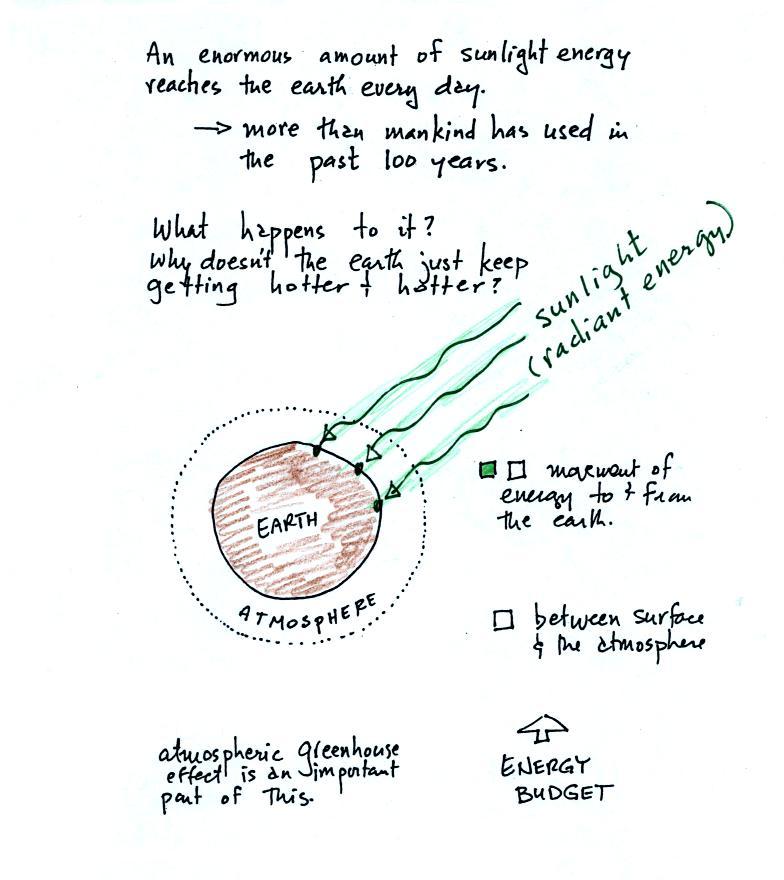
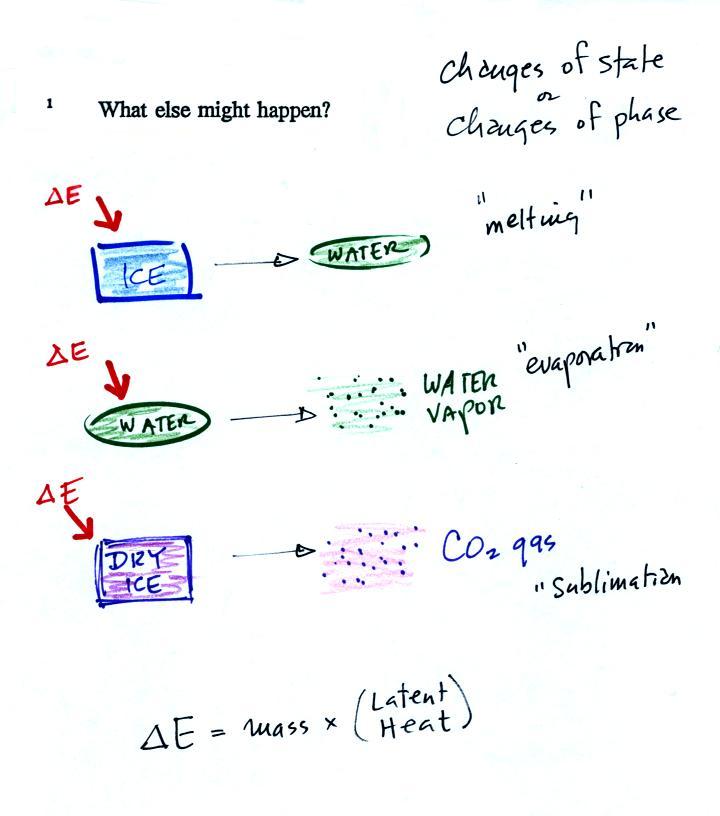
The equation at the bottom of the
figure above allows you to
calculate how much energy is required to melt ice or evaporate water or
sublimate dry ice. You multiply the mass by the latent heat, a
variable that depends on the particular material that is changing
phase.
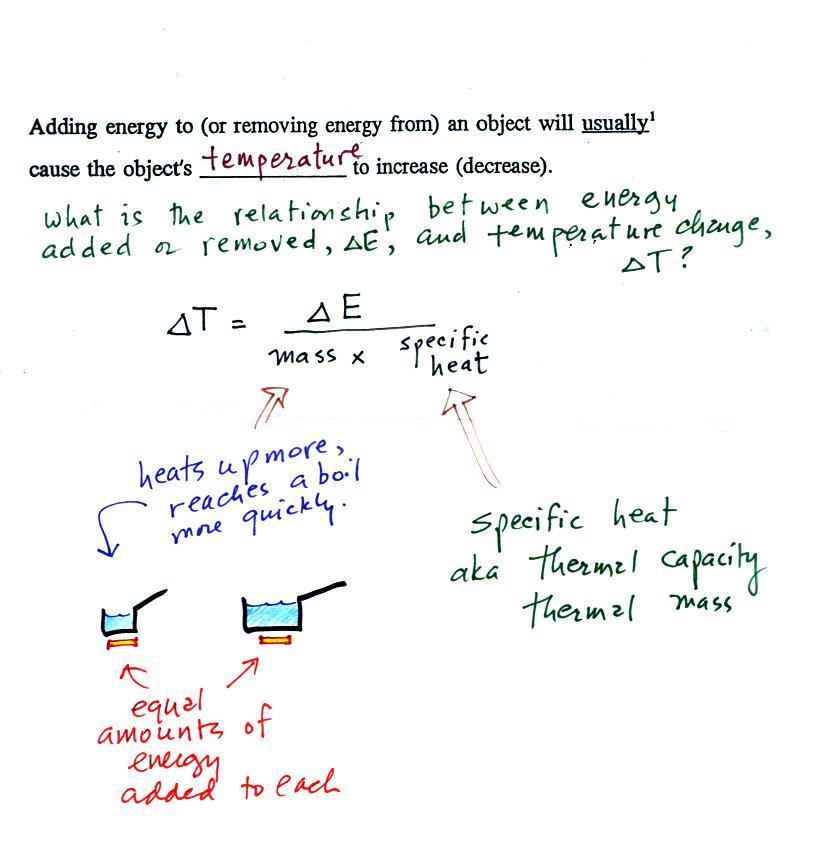
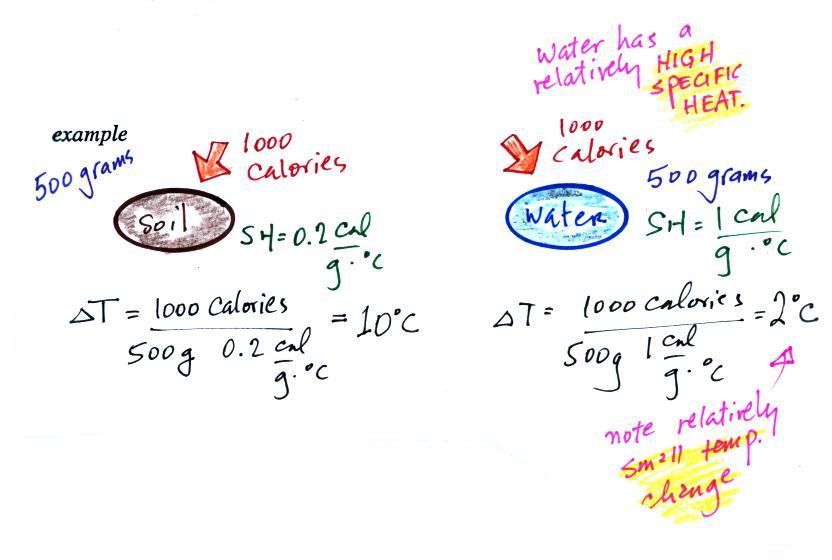
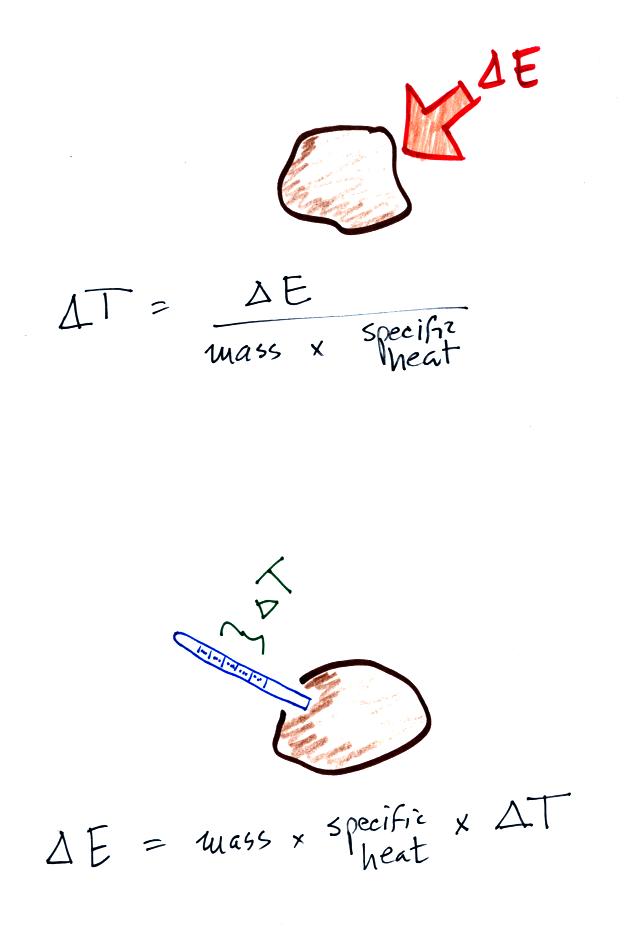
If you add energy to or remove energy from an object, the
object
will usually change temperature. You can calculate the
temperature change if you know the object's mass and its specific
heat. That's the equation we used in the example calculation
earlier.
We will be using the equation next in a slightly different way in a
class
experiment/demonstration. The equation is also used Latent Heat
of Fusion of Ice
Experiment included in this week of the course. We will measure
temperature change and
use that to
determine the amount of energy gained or lost by an object.
The experiment is normally conducted by a handful of student
volunteers in the classroom version of the course. The object is
to
measure the latent heat of
vaporization of liquid nitrogen. That just means measuring the
amount of energy needed to evaporate a gram of liquid nitrogen.
In the Latent Heat of Fusion of Ice Experiment you will measure the
energy needed to melt one gram of ice. Before beginning the
experiment, a sealed envelope containing the known latent heat of
vaporization of nitrogen is given to a student in the class.
Some data from an actual experiment are shown above. The
various steps of the experiment are explained below.
(a)
Some room temperature water poured into a styrofoam cup weighed
104.4
g. The cup itself weighed 3.4 g, so we had 101.0 g of water.
(b)
The water's temperature was 21.0 C (room temperature).
(c)
40.0 g of liquid nitrogen was poured into the cup of water.
It takes energy to turn liquid nitrogen into nitrogen gas.
The needed energy came from the water. This flow of energy is
shown in the middle figure above. We assumed that because the
experiment is performed in a styrofoam cup that there is no energy
flowing between the water in the cup and the surounding air.
(d)
After the liquid nitrogen had evaporated we remeasured the water's
temperature. It had dropped to 1.0 C. That is a
temperature drop of 21.0 - 1.0 = 20.0 C.
Because we knew how
much water we started with, its temperature drop, and water's specific
heat we can calculate how much
energy was taken from the water.
101.0 g x 20 C x 1 cal/g C
= 2020 calories
We then divide that number by the amount of liquid nitrogen that was
evaporated.
2020 calories / 40 grams = 50.5
calories per gram
A
trustworthy student in the class informed us that
the known value is 48 cal/g. The student's measurement was pretty
darn close to that value.