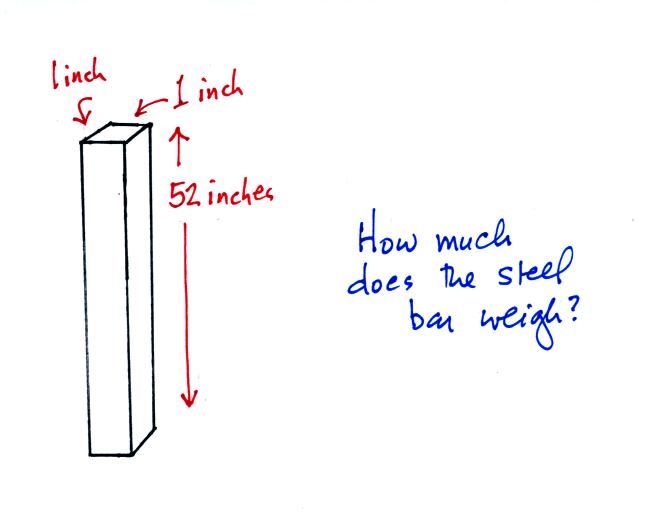
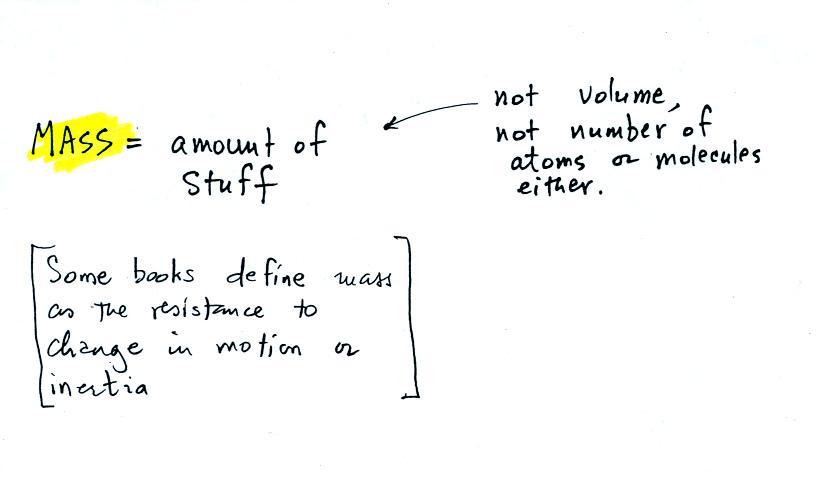
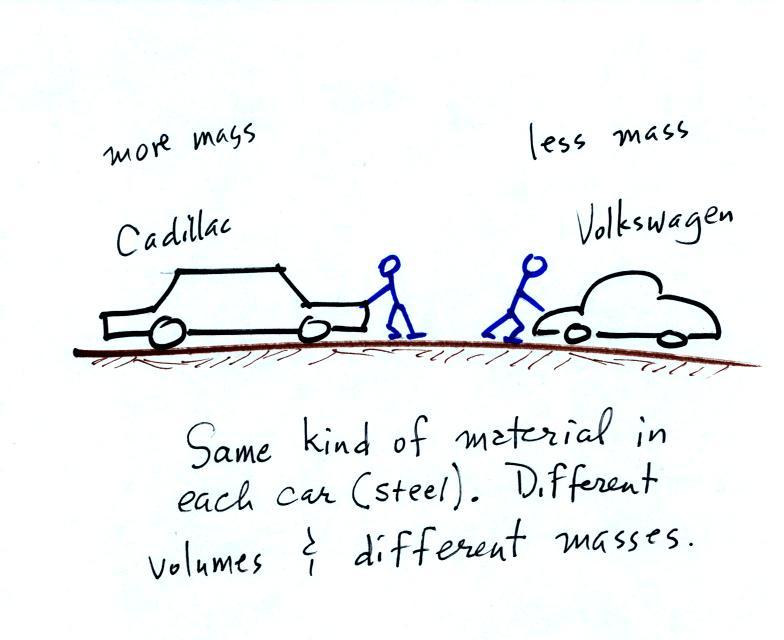
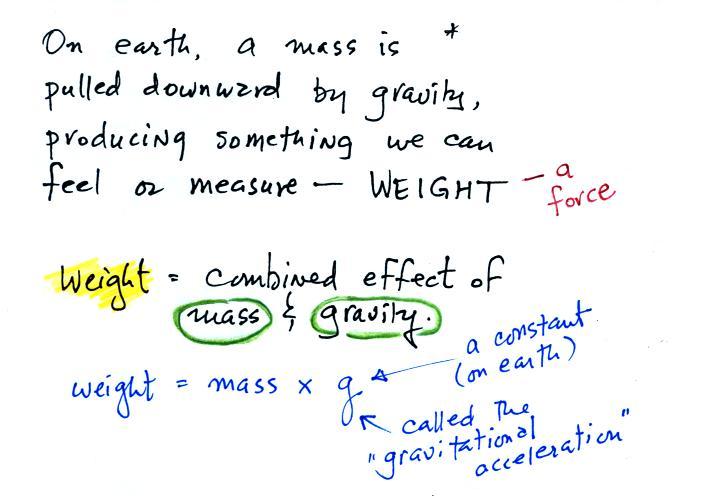
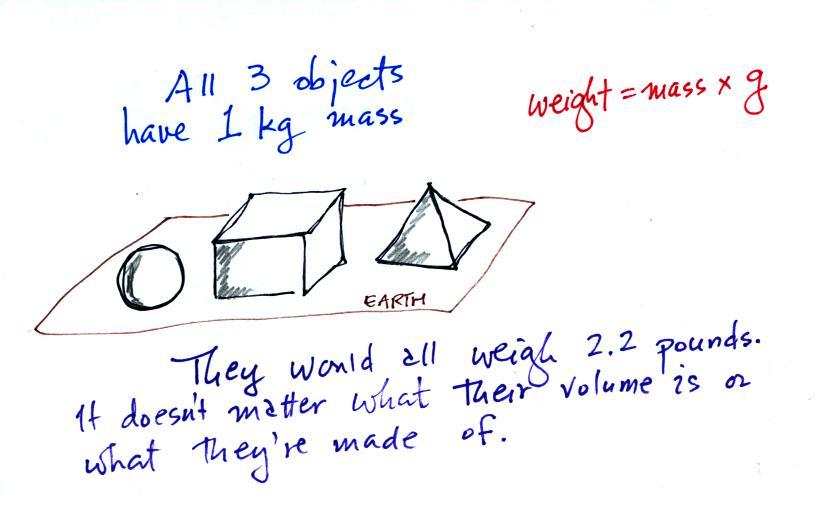
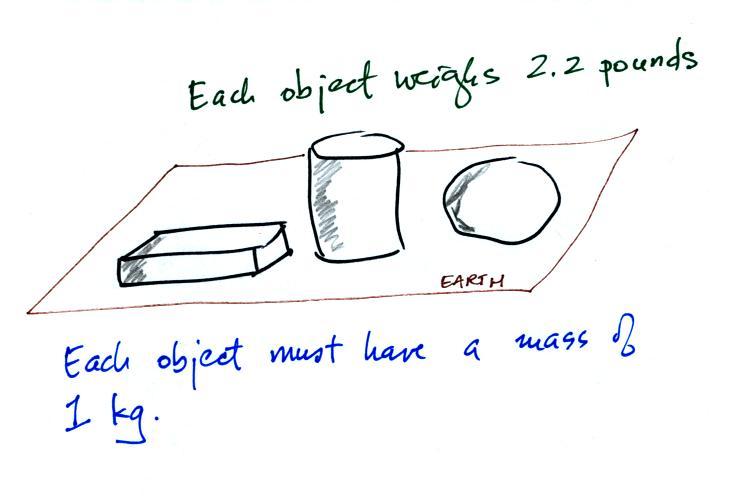
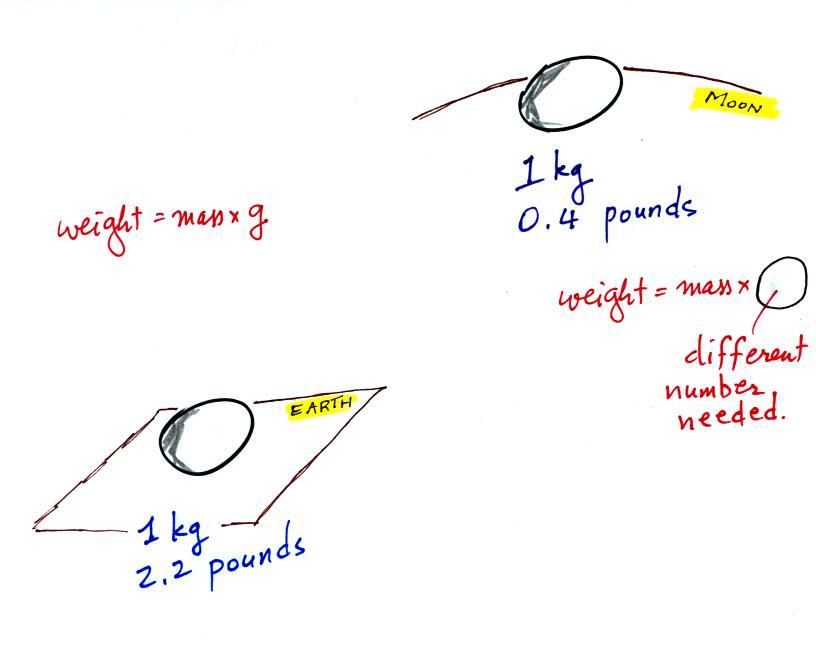
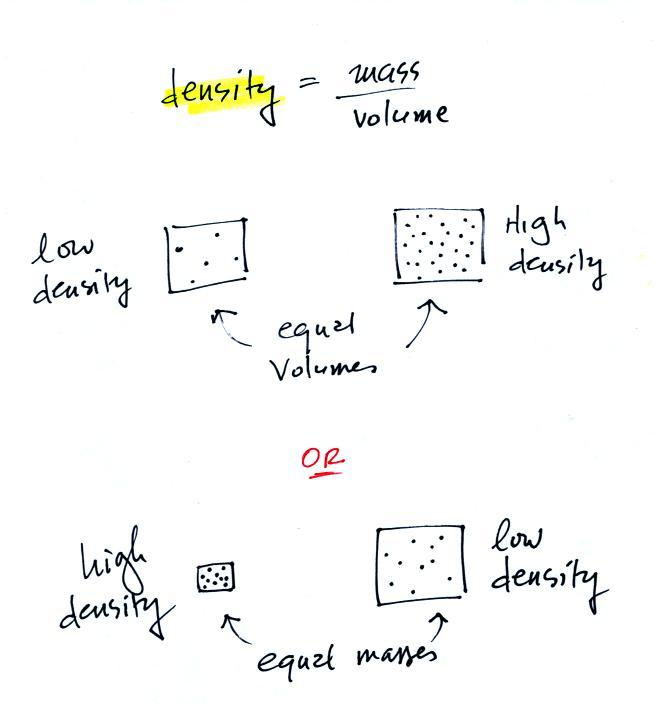
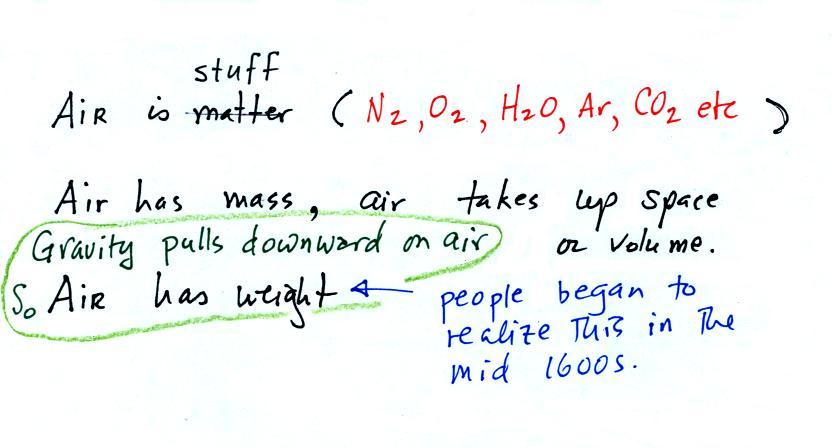
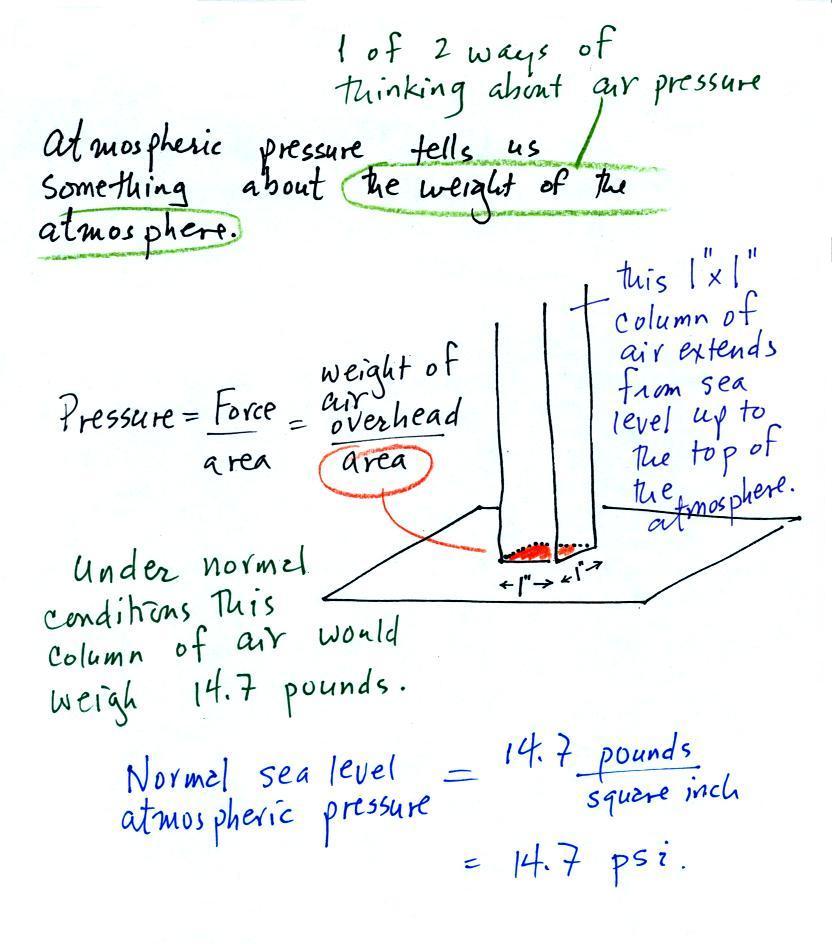
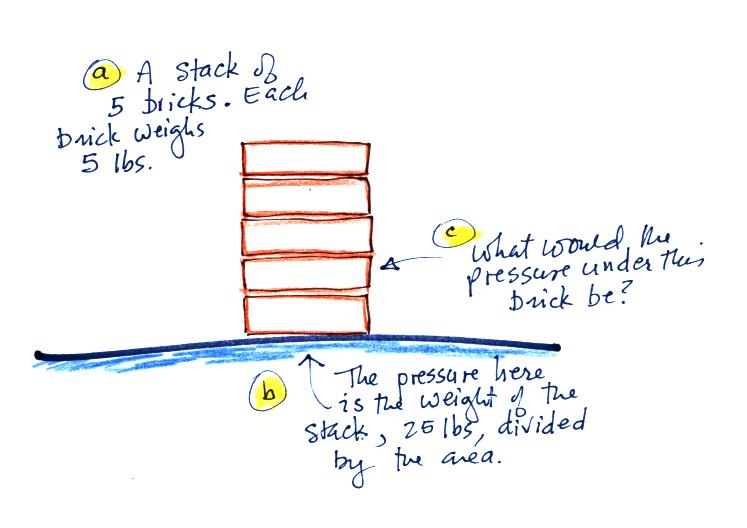
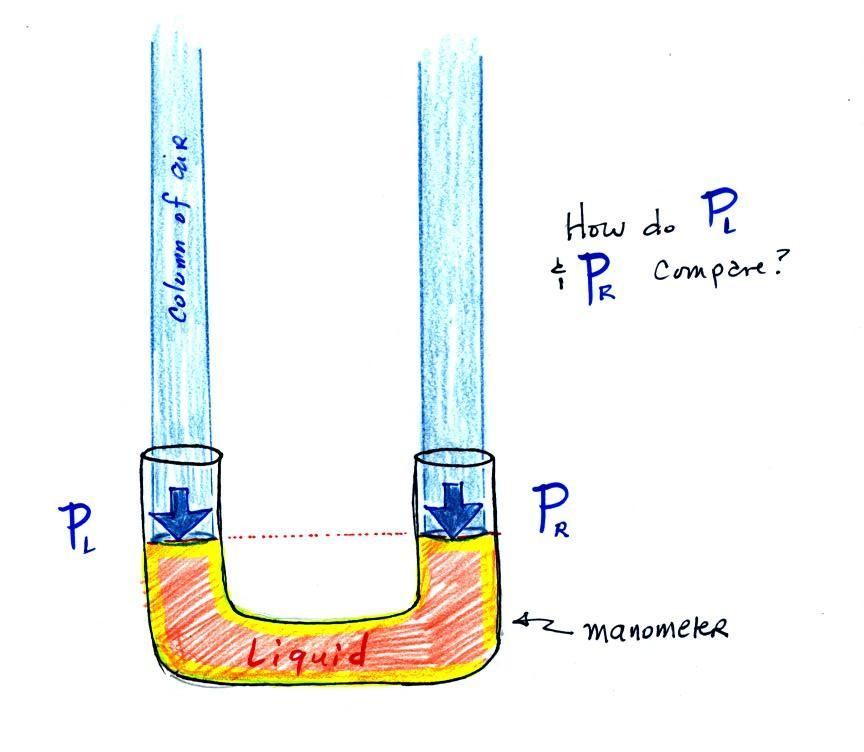
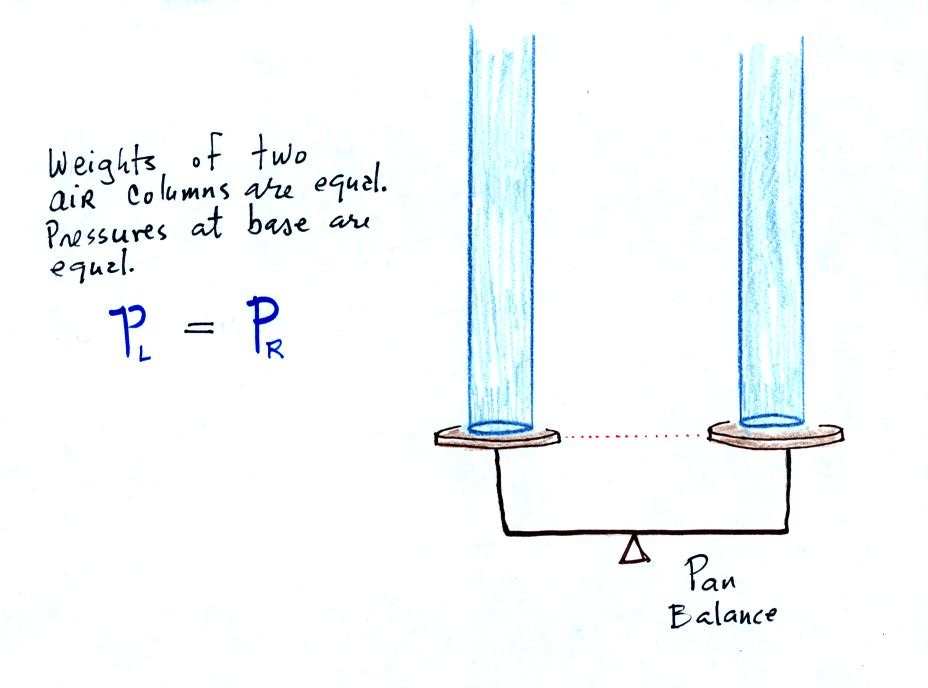
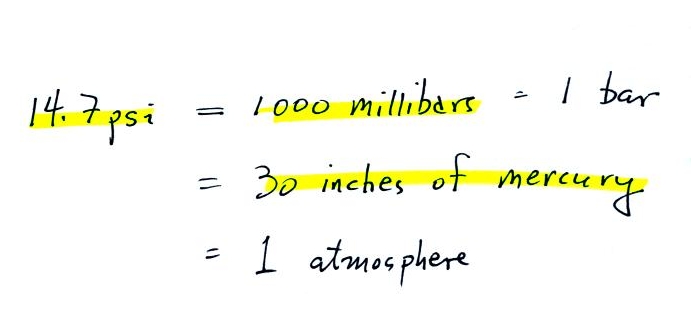The instrument in the left figure
above ( a u-shaped
glass
tube filled with a
liquid of some kind) is actually called a manometer and can be used to
measure pressure
difference. The
two ends of the tube are open so that air can get inside and air
pressure can press on the liquid. Given that the liquid levels on
the two sides of the manometer
are equal, what could you about PL and PR?
The liquid can slosh back and
forth just like the pans on a balance can move up and down. A
manometer really behaves just like a pan balance (pictured at
right). Because the two pans are in balance, the two columns of
air have the same weight.
PL and PR
are equal (note
you don't really know what either pressure is, just that they are
equal).
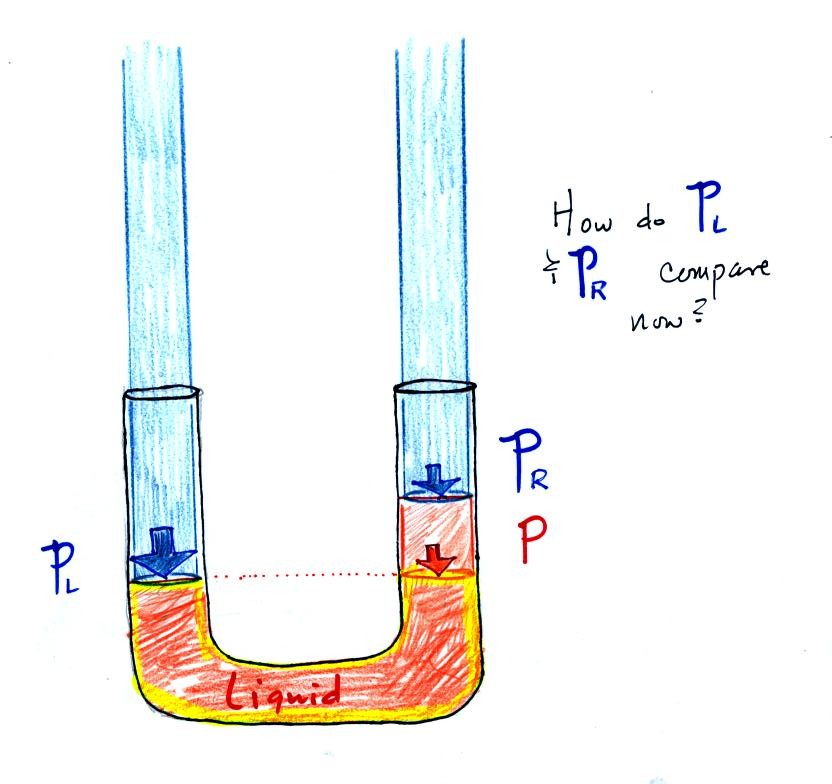 |
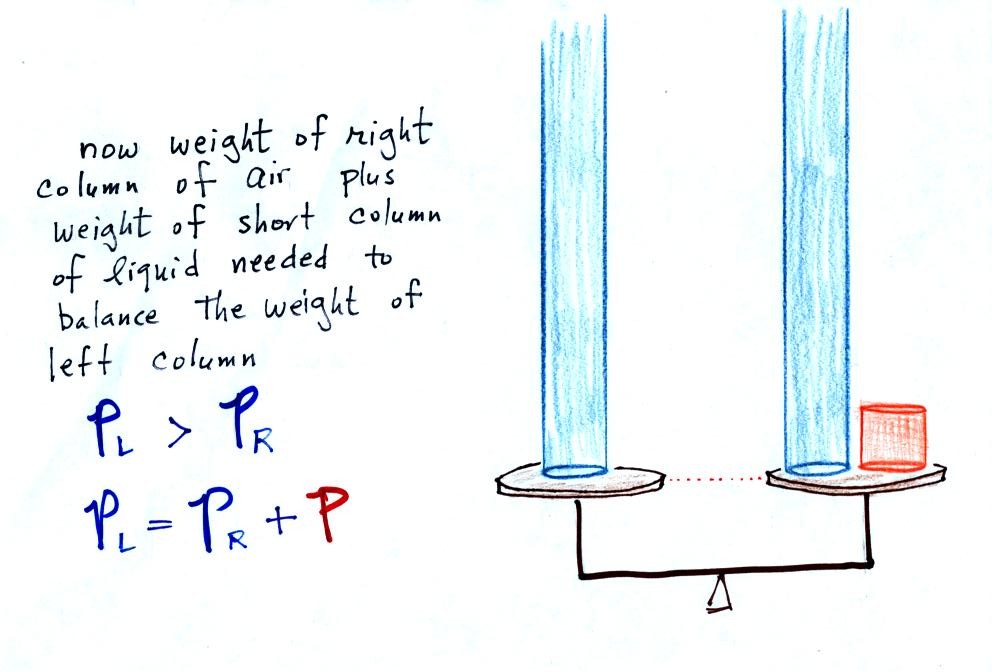 |
Now
the
situation is a little
different,
the
liquid levels
are no
longer equal. You probably realize that the air pressure on the
left, PL, is a little higher than the air pressure on the
right,
PR. PL is now being balanced by PR
+ P acting together. P
is the pressure produced by the weight of the extra fluid on the right
hand side of
the manometer (the fluid that lies above the dotted line). The
height
of
the
column
of
extra
liquid
provides
a
measure
of
the difference between PL and PR.
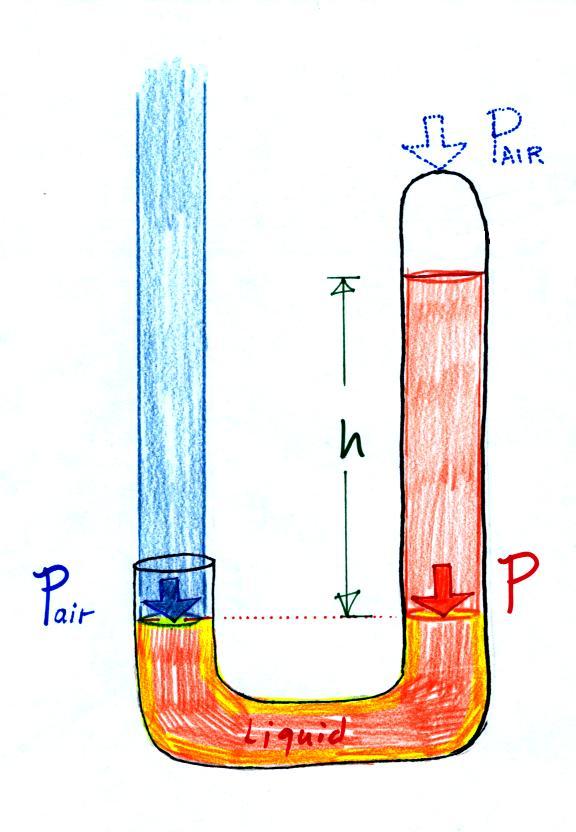
Next we will just go and close off
the right hand side of the
manometer.
|
|
Air pressure can't get into the
right tube any
more. Now at the level of the dotted line the balance is between
Pair and P (pressure by the extra liquid on the
right). If
Pair
changes, the height of the right column, h, will
change. You now have a barometer, an instrument that can measure
and monitor the atmospheric pressure. (some of the letters were cut off
in the upper right portion of the left figure, they should read "no air
pressure")
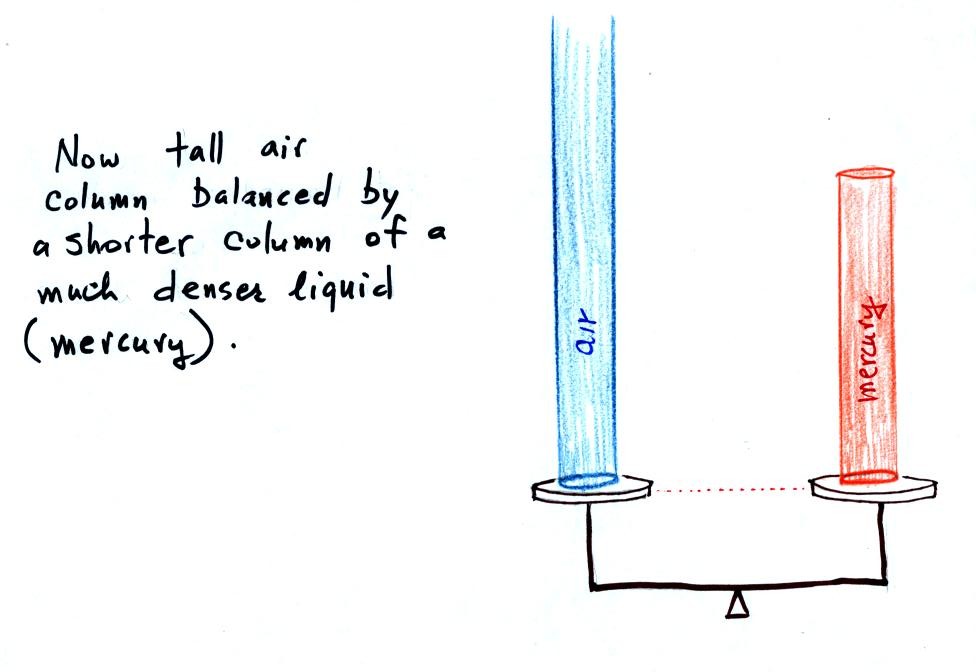
Barometers like this are usually
filled with mercury. Mercury is
a liquid. You need a liquid that can slosh back and forth in
response to changes in air pressure. Mercury is also very dense
which
means the barometer won't need to be as tall as if you used something
like water. A water barometer would need to be over 30 feet
tall. With mercury you will need only a 30 inch tall column to
balance the weight of the atmosphere at sea level under normal
conditions (remember the 30 inches of mercury pressure units mentioned
earlier). Mercury also has a low rate of
evaporation so you don't have much mercury gas at the top of the right
tube (it is the mercury vapor that would make a mercury spill in the
classroom dangerous).

Here is a more conventional
barometer design.
The bowl of
mercury is usually covered in such a way that it can sense changes in
pressure but not evaporate and fill the room with poisonous mercury
vapor.
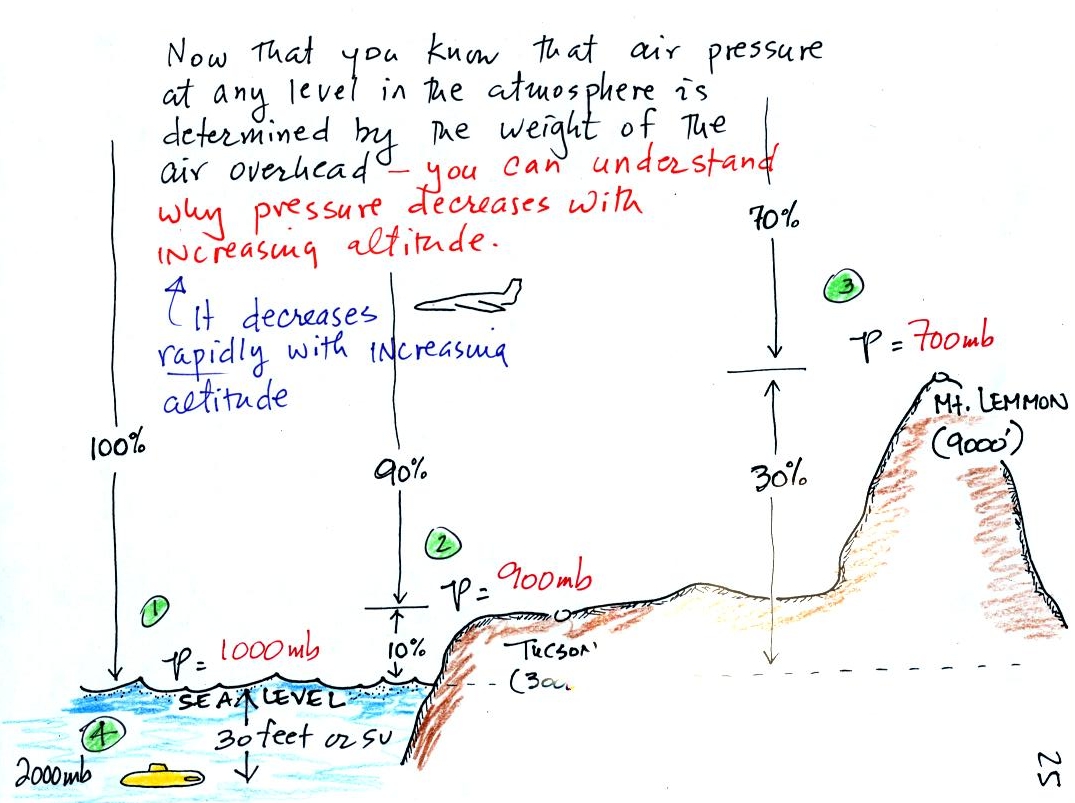
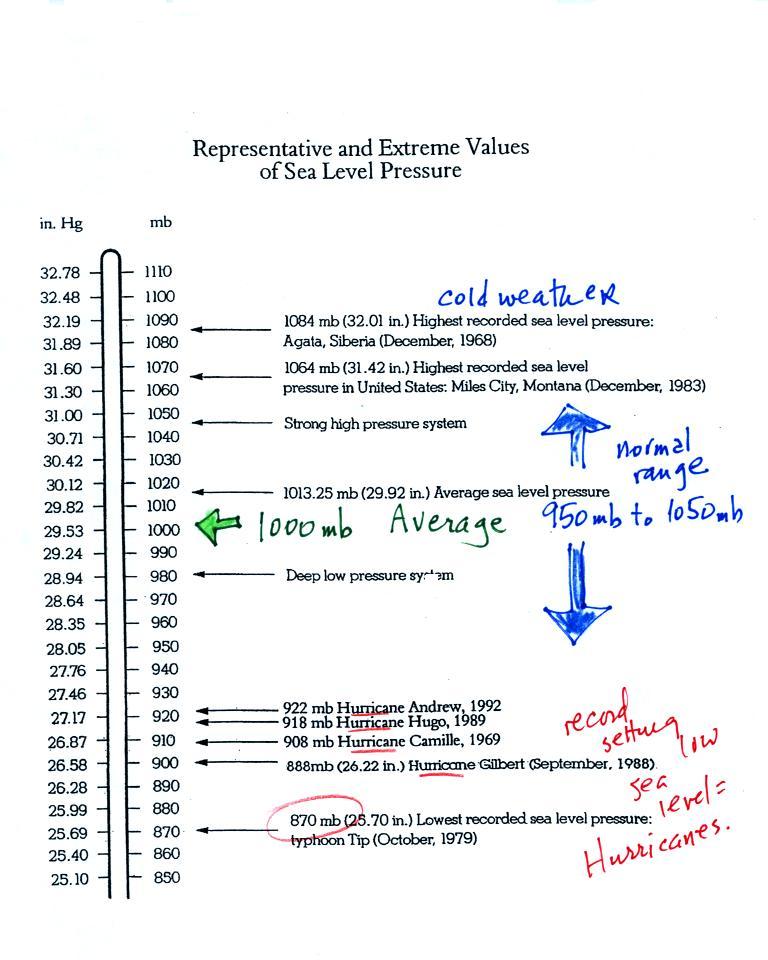
The figure above first
shows average sea level pressure values. 1000 mb or 30 inches of
mercury are close enough in this class.
Sea level pressures
usually fall between 950 mb and 1050 mb.