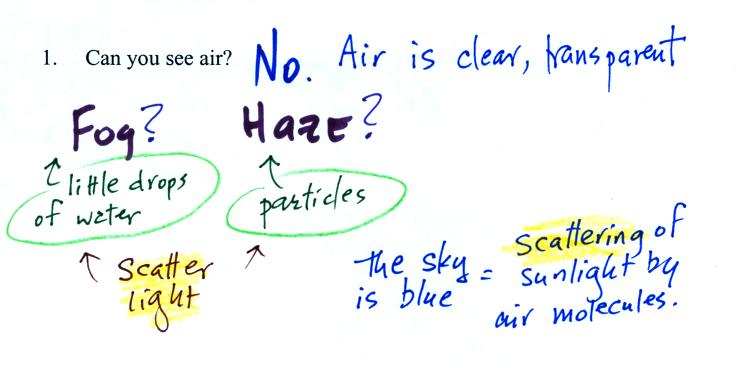
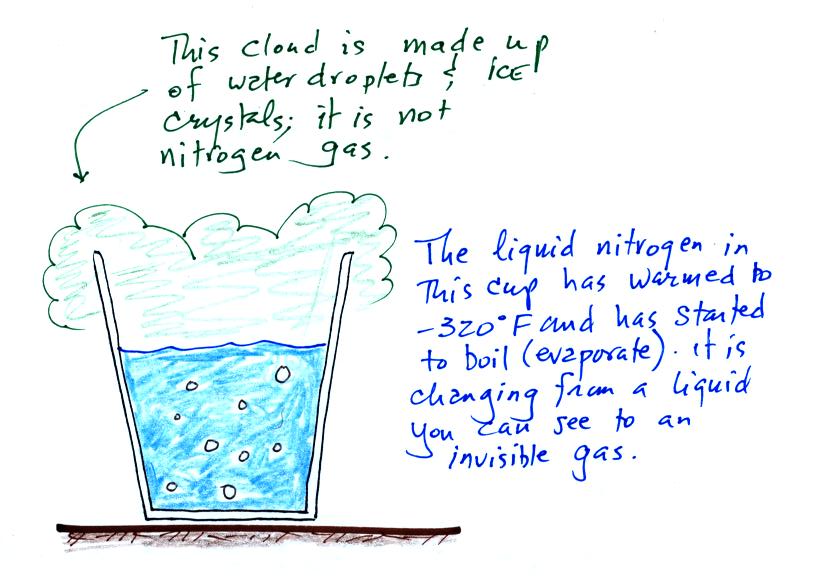
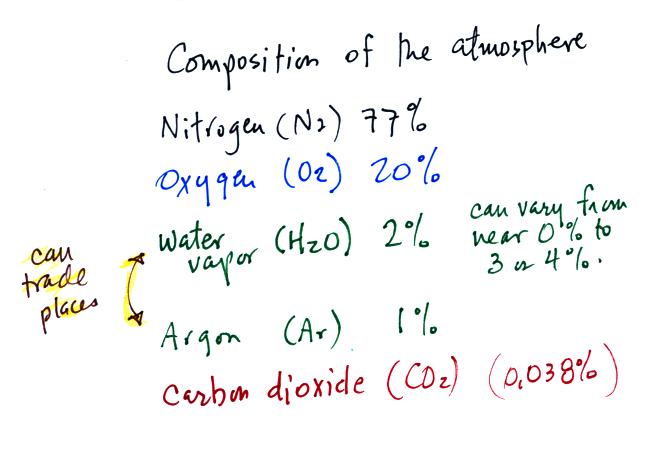
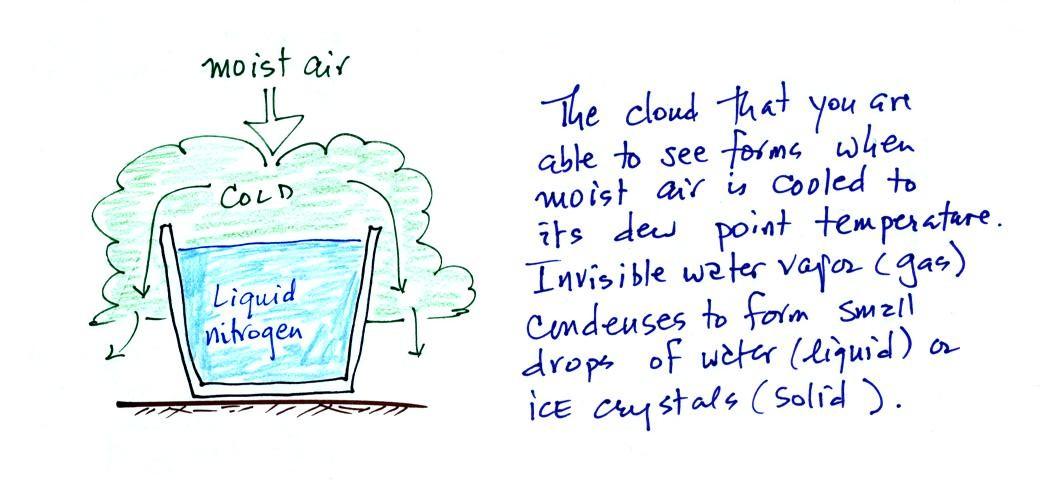
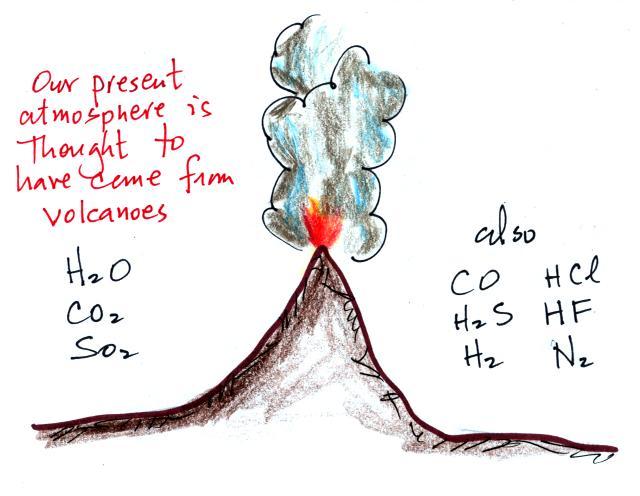
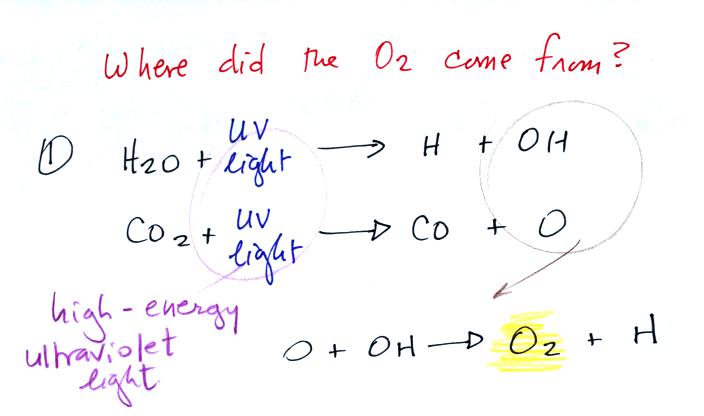
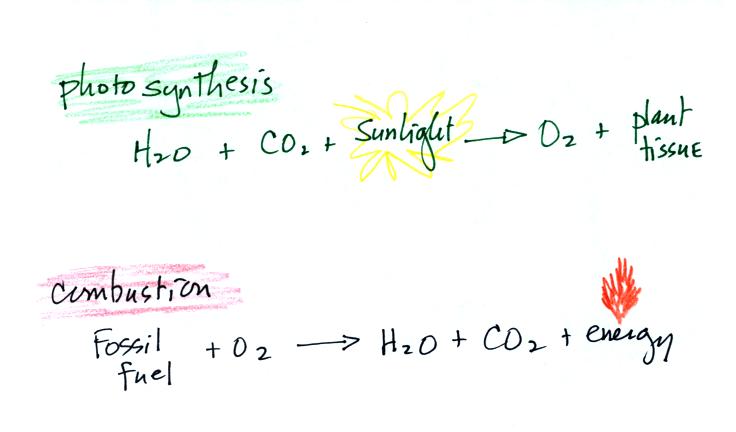
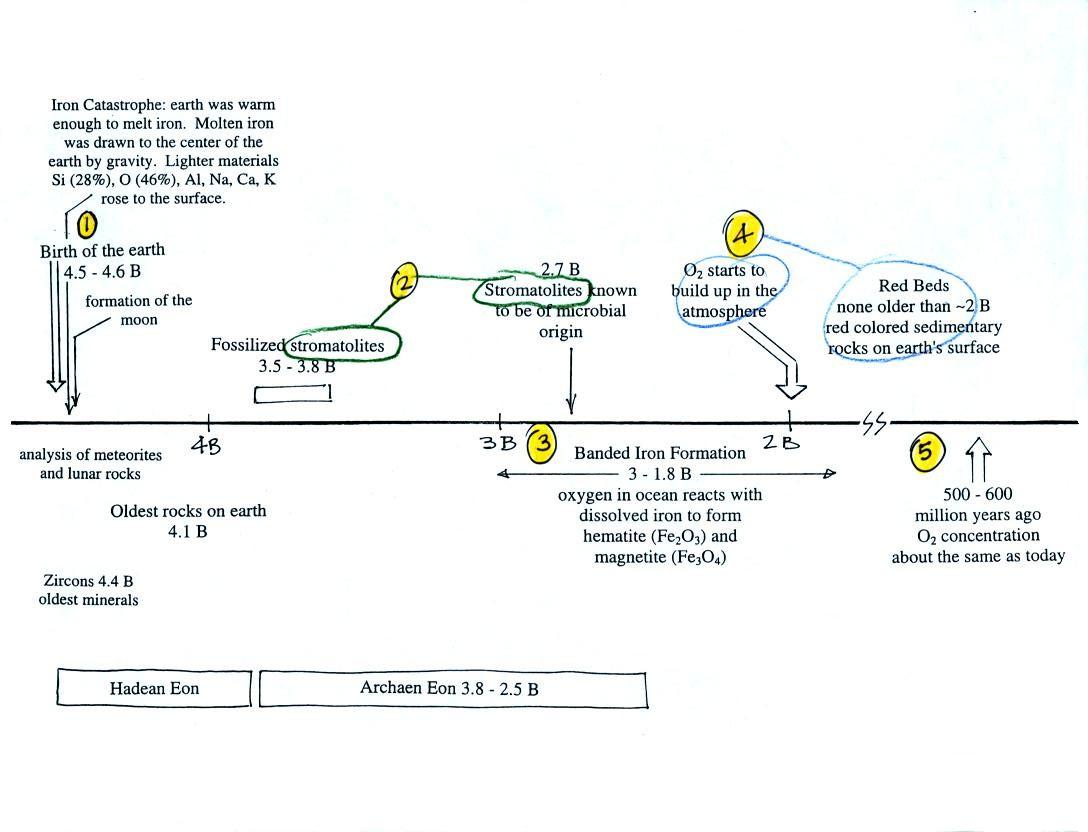
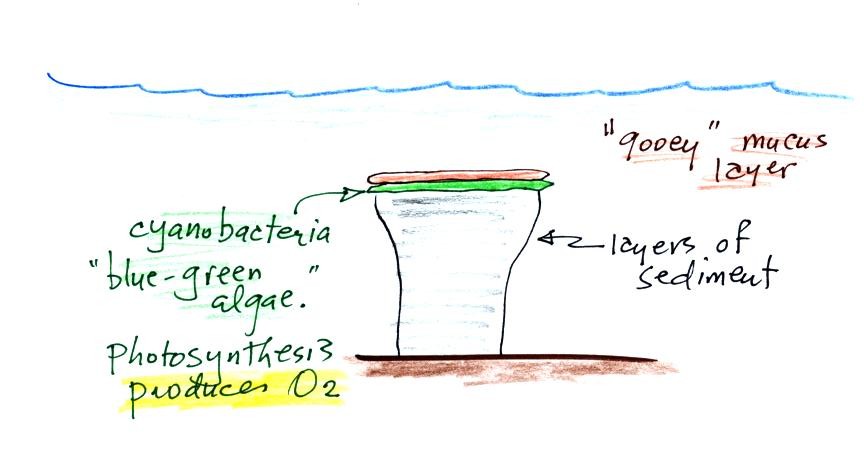
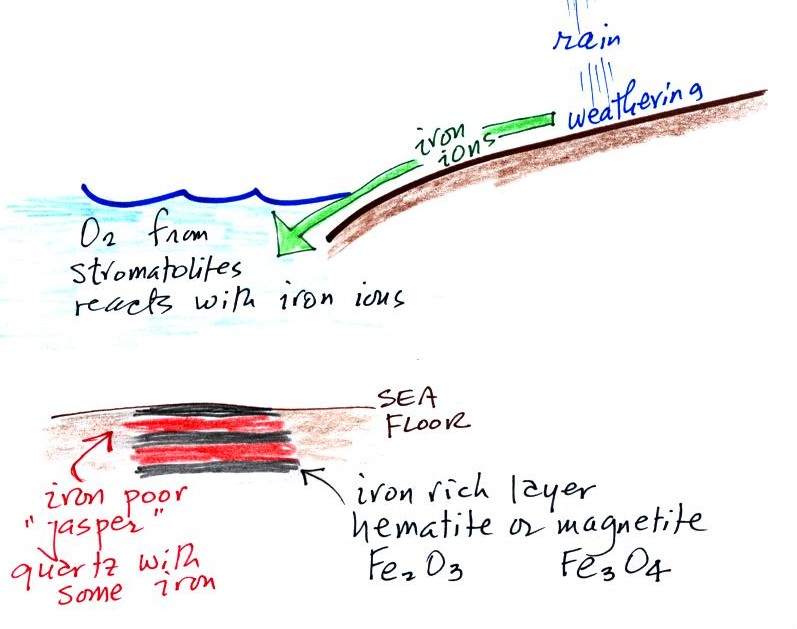
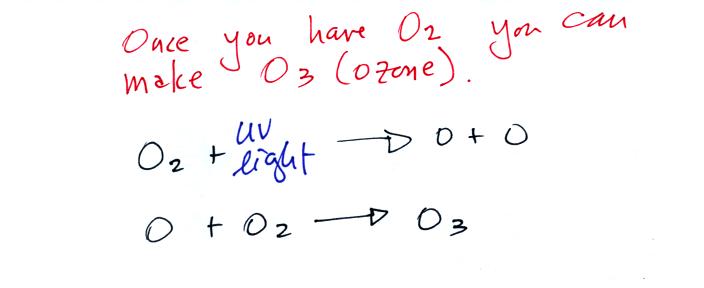Living stromatolites are found
in a
few locations today. The picture above is from Coral Bay Australia, located on
the
western tip of the continent. The picture was probably taken at
low tide, the stromatolites would normally be covered with ocean water.
Once cyanobacteria began to produce
oxygen in ocean water, the oxygen reacted with dissolved iron (iron
ions in the figure below) to form hematite or magnetite. These
two minerals precipitated out of the water to form a layer on the sea
bed.
Periodically the oxygen production would decrease or stop (rising
oxygen levels might have killed the cyanobacteria or seasonal changes
might have slowed the photosynthesis). During these times of low
dissolved oxygen concentrations, layers of a different mineral, jasper,
would form on the
ocean bottom. Eventually the cyanobacteria would recover, begin
producing oxygen again, and a new layer of hematite or magnetite would
form. The rocks that resulted, containing alternating layers of
black hematite or magnetite and red layers of jasper are known as the
banded
iron formation (Point 3).
A polished slice of banded iron rock is shown below.
Actual rock
samples are fairly impressive because they are heavy
(they contain a lot of iron) and are also 2 - 3 billion years old!