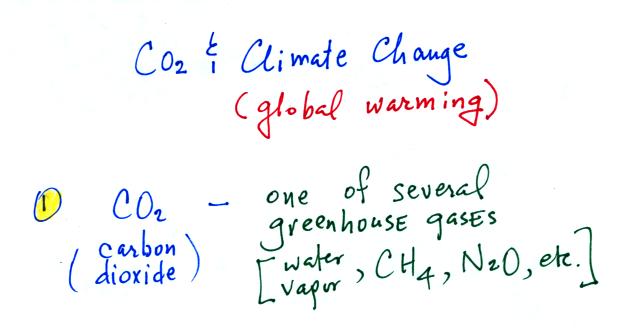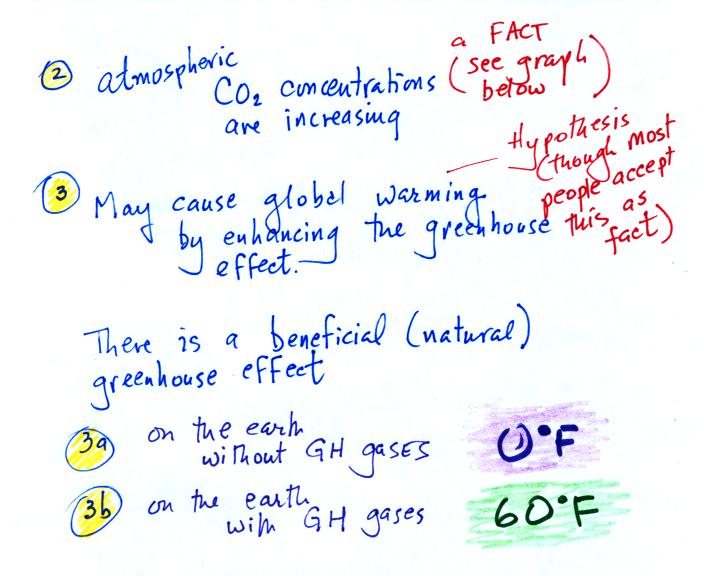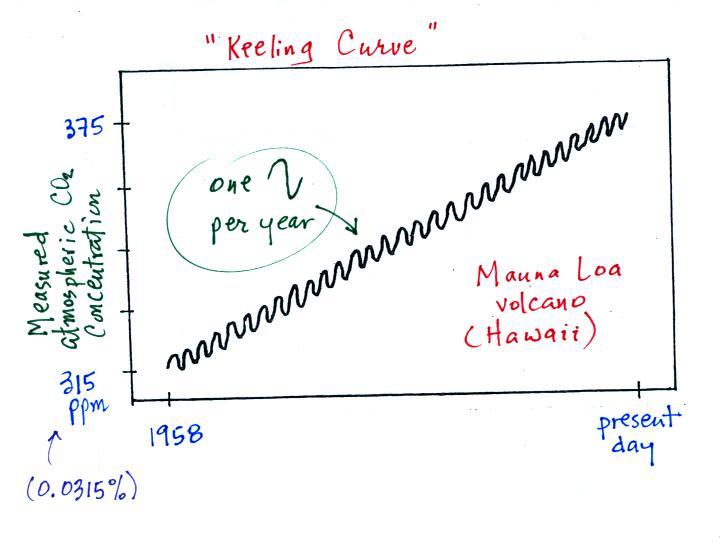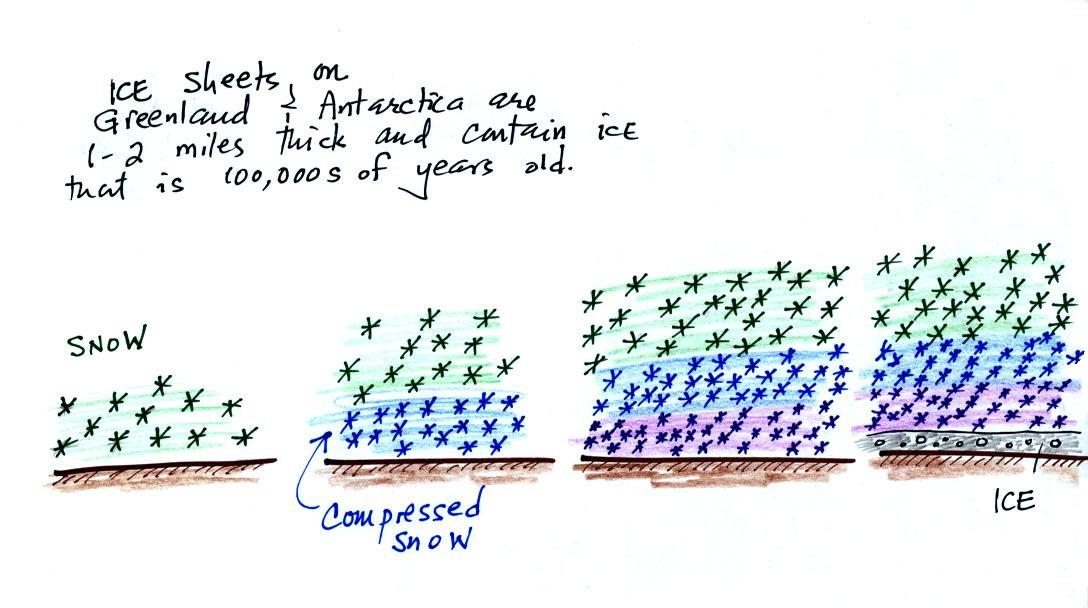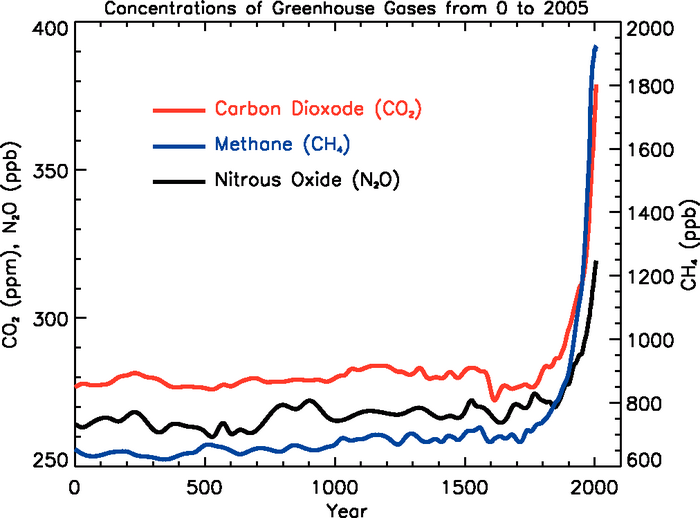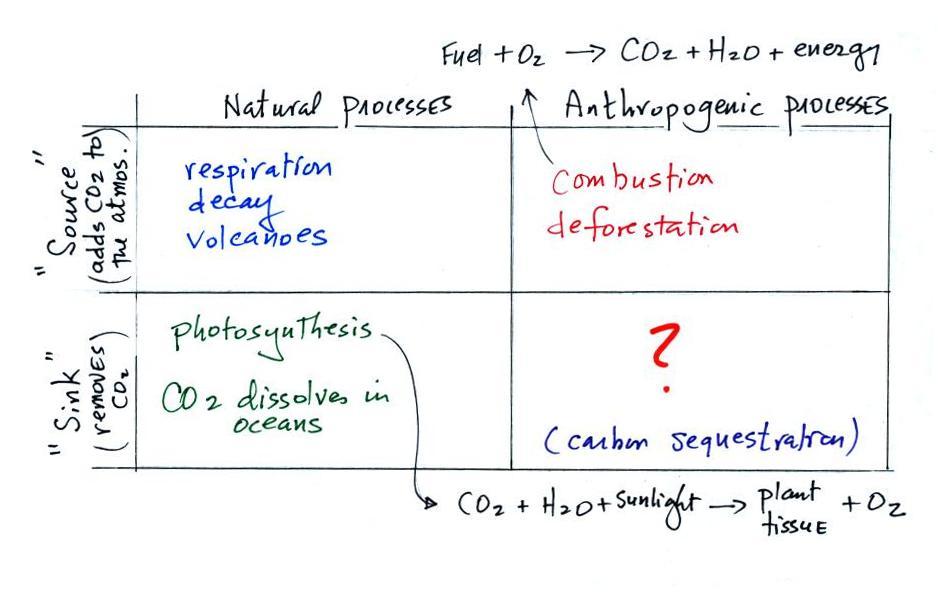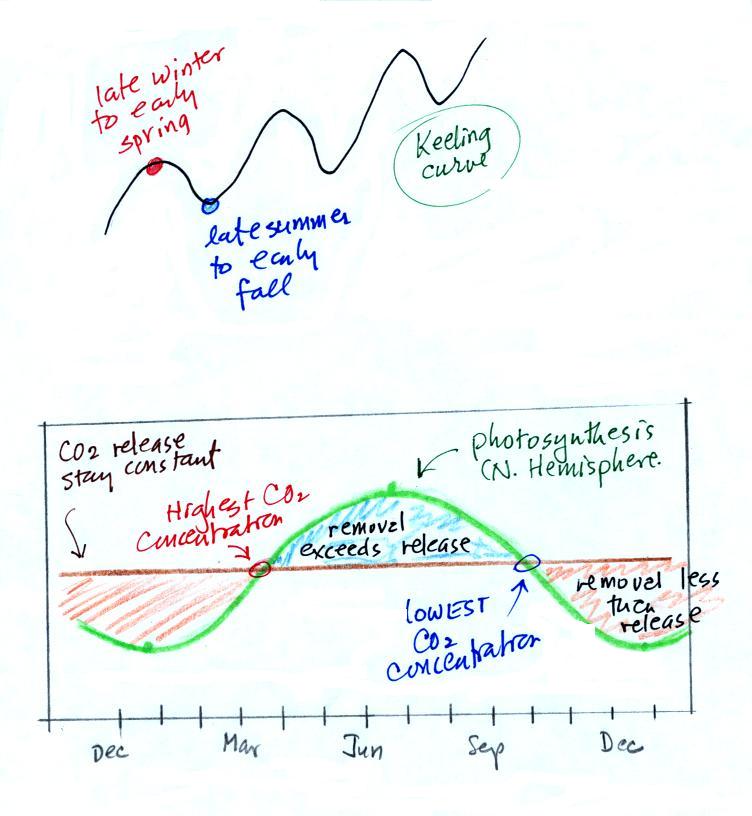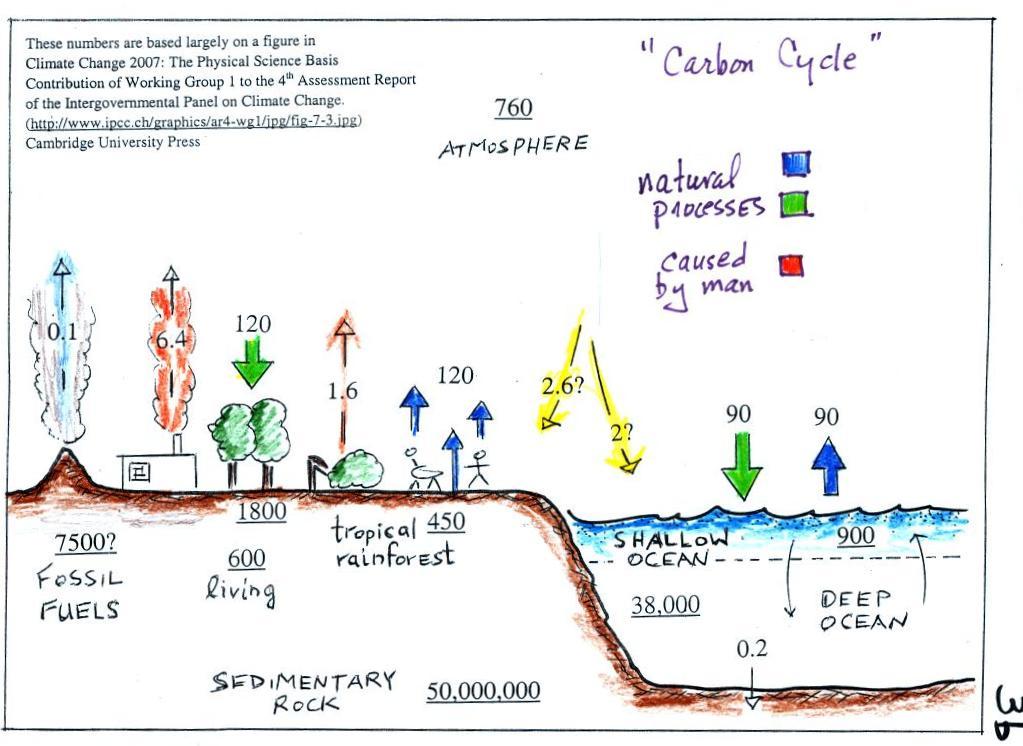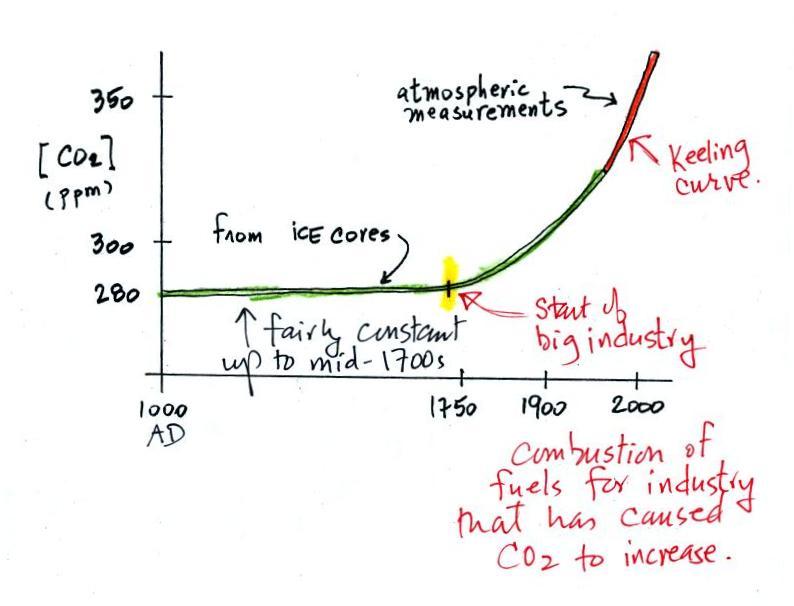
The green portion of this curve
shows determined from the ice cores. The more
recent Keeling curve measurements are shown in red at the right end of
the curve. Atmospheric CO2 concentration was fairly
constant at about
280 ppm
between 1000 AD and the mid-1700s when it started to increase.
The
start
of rising CO2 coincides with the
beginning of the
"Industrial
Revolution." Combustion of fossil fuels needed to
power factories began to add
significant amounts of CO2
to the
atmosphere.