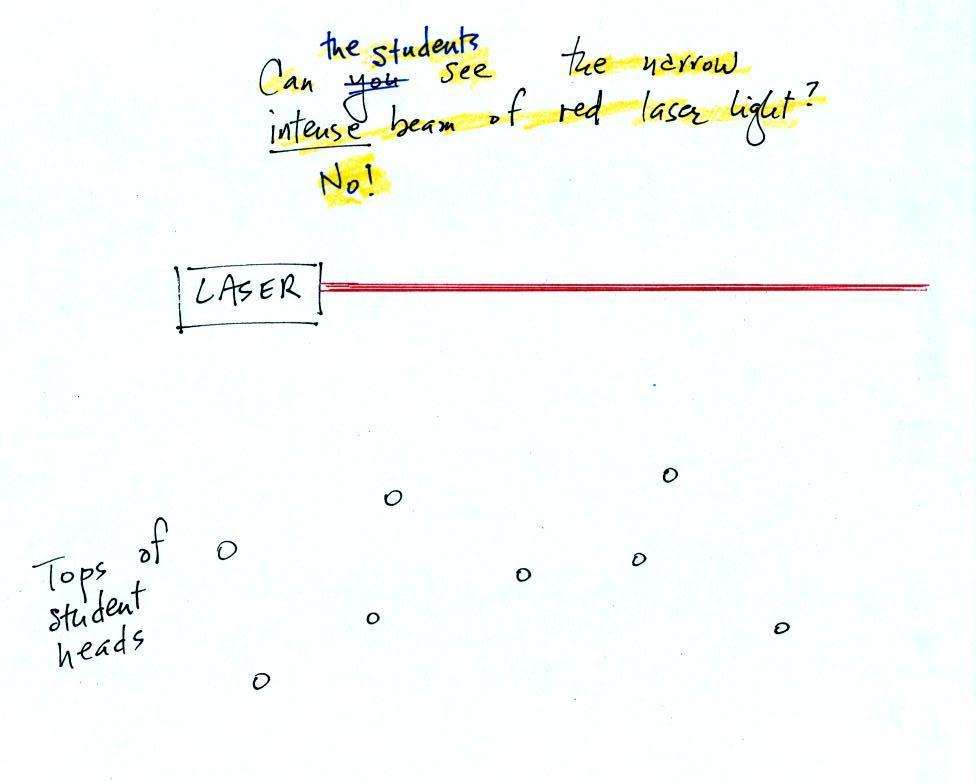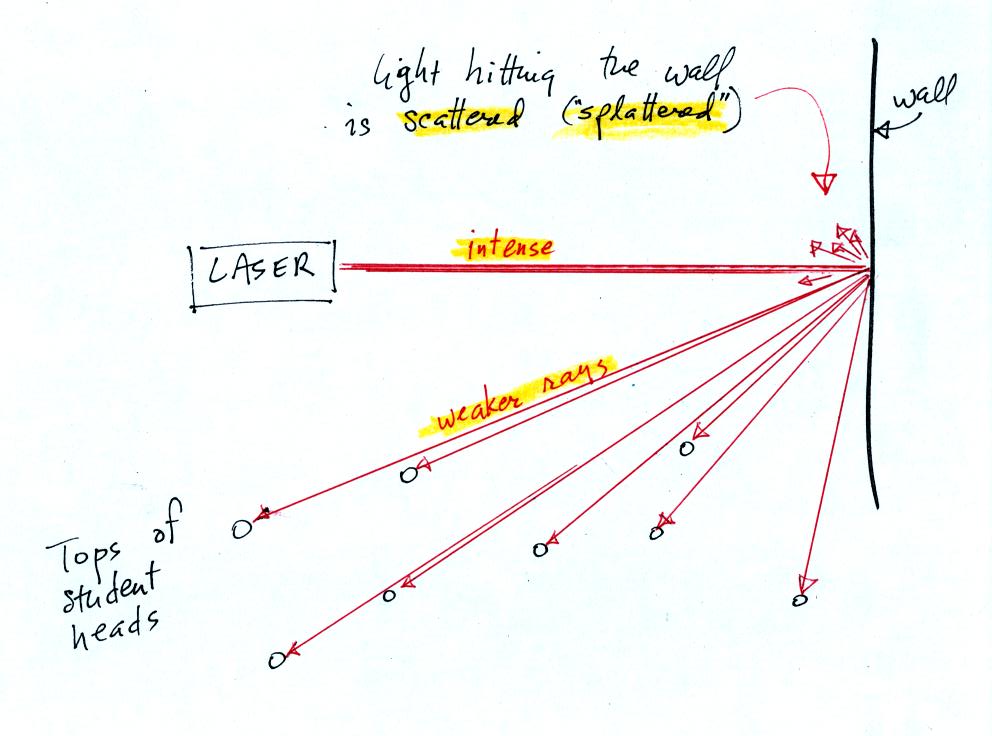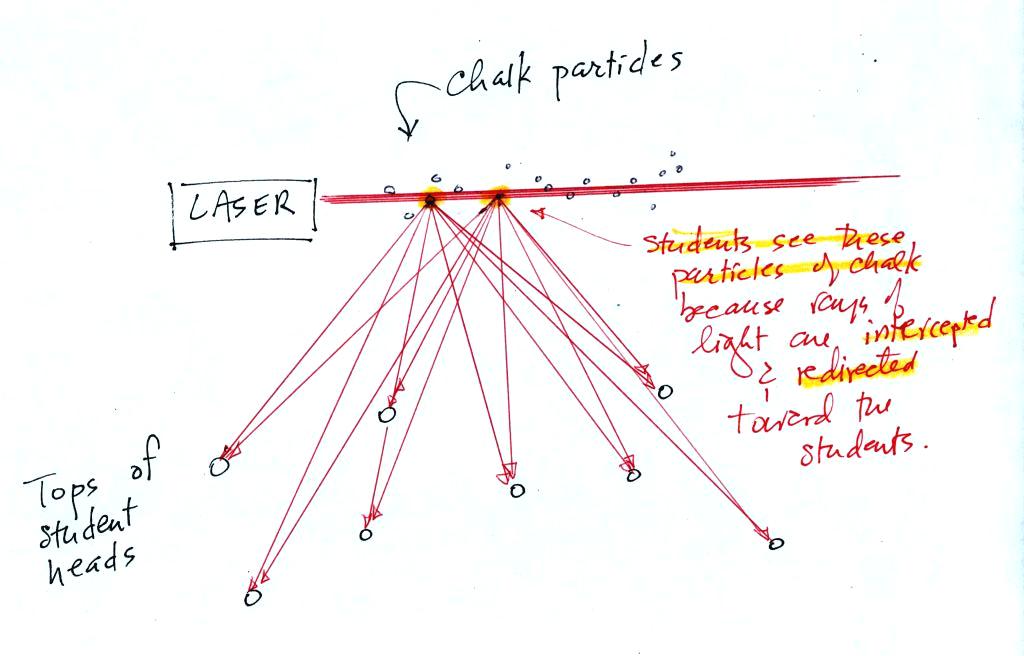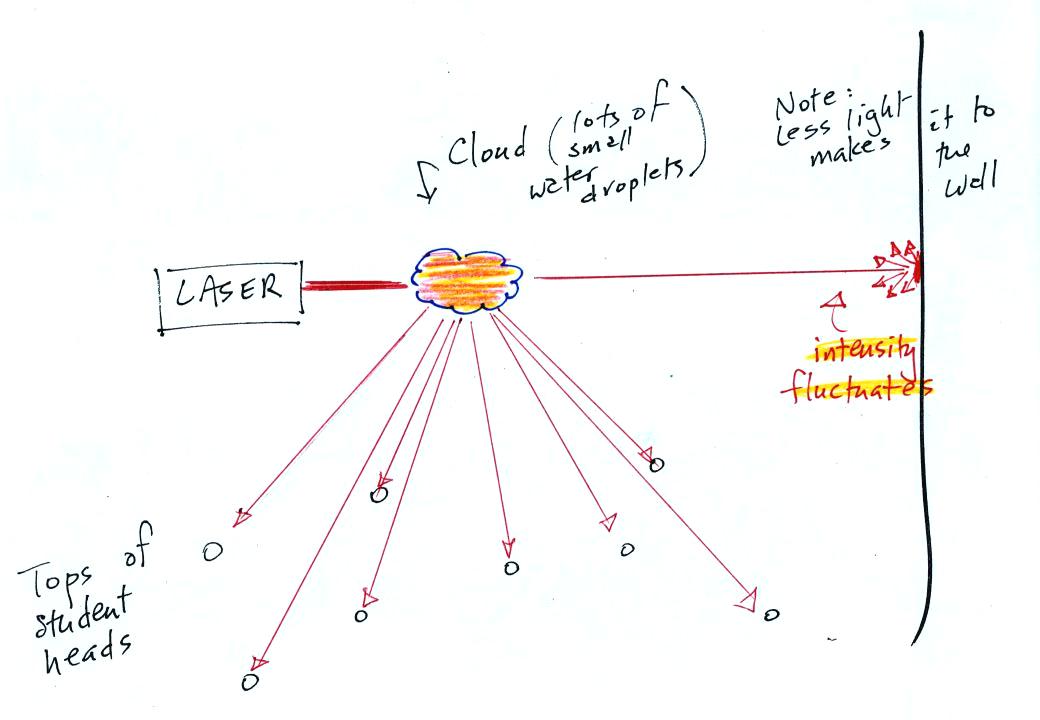
The laser light really lit up and
turned the small patches of
cloud
red. The cloud did a very good job of scattering laser light. So
much light was scattered
that the spot on the wall fluctuated in intensity (the spot dimmed when
lots of
light was being scattered, and brightened when not as much light was
scattered).
A comment that may not have been mentioned
in
class (if it was mentioned it certainly wasn't emphasized).
Air molecules are able
to scatter light too, just like cloud droplets. Air molecules are
much smaller than cloud droplets and don't scatter much light.
That's why you weren't able to see light being scattered by air before
we
put chalk particles or cloud droplets into the beam. Outdoors you
are able to see sunlight (much more intense than the laser beam used in
the class demonstration) scattered by air molecules. Sunlight is
white and is made up of violet, blue, green, yellow, orange, and red
light. Air molecules have an unusual property: they scatter the
shorter wavelengths (violet, blue, green) much more readily than the
longer wavelength colors in sunlight (yellow, orange, and red).
When you look away from the sun and look at the sky, the blue color
that you see are the shorter wavelengths in sunlight that are being
scattered by air molecules.