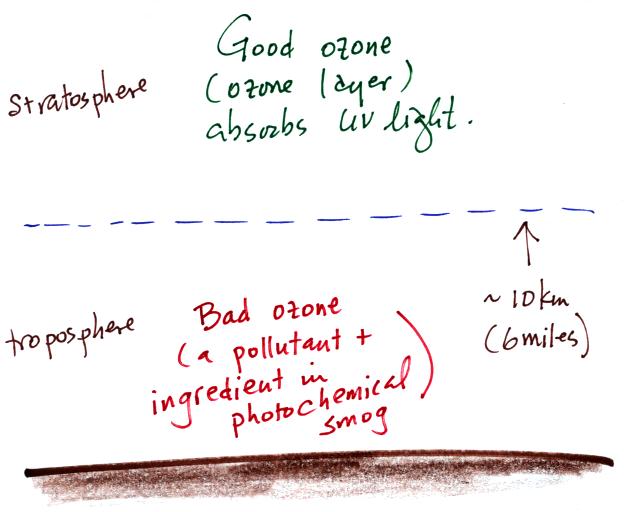
Ozone found in the stratosphere
absorbs dangerous high-energy ultraviolet light and has a benefical
role. Ozone in the troposphere (at ground level) is a
pollutant. It is a toxic gas and is also a key ingredient in Los
Angeles type smog (aka photochemical smog).
The purpose of the demonstration was to make some photochemical smog. We first needed some ozone. The reactions that produce ozone in the stratosphere are very simple.
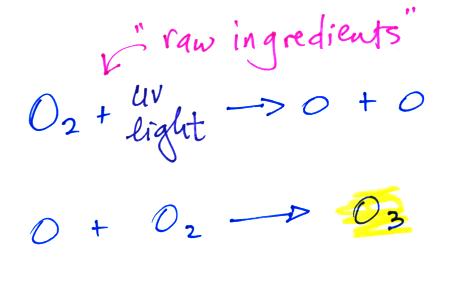
The purpose of the demonstration was to make some photochemical smog. We first needed some ozone. The reactions that produce ozone in the stratosphere are very simple.

Molecular oxygen (O2) absorbs
ultraviolet (UV) light and is split into two oxygen atoms. The
oxygen atoms then react with oxygen molecules to make ozone (O3).
This is the process that we will use to make some ozone for the smog
demonstration. We'll use oxygen in the air and a small lamp that
emits UV light (the bulb was placed inside a glass flask that was
covered with a black cloth to insure that people in the classroom
weren't exposed to dangerous UV light.
The next step in the demonstration was to introduce some hydrocarbons into the flash so that it could react with the ozone. Hydrocarbons are compounds that contain hydrogen and carbon (I mentioned they also contained oxygen in class, apparently that isn't correct). In the demonstration some lemon peel was put into the flask. The lemony smell from the lemon peels is a hydrocarbon.
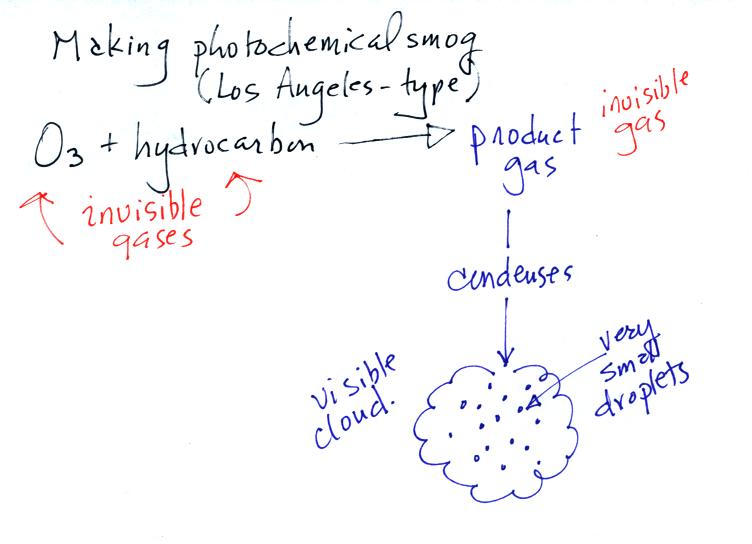
The next step in the demonstration was to introduce some hydrocarbons into the flash so that it could react with the ozone. Hydrocarbons are compounds that contain hydrogen and carbon (I mentioned they also contained oxygen in class, apparently that isn't correct). In the demonstration some lemon peel was put into the flask. The lemony smell from the lemon peels is a hydrocarbon.

The reaction between the ozone and
the lemon peel produces some product gas (invisible). The product
gas condenses however and forms small drops or particles that scatter
light. At this point a smog cloud becomes visible.
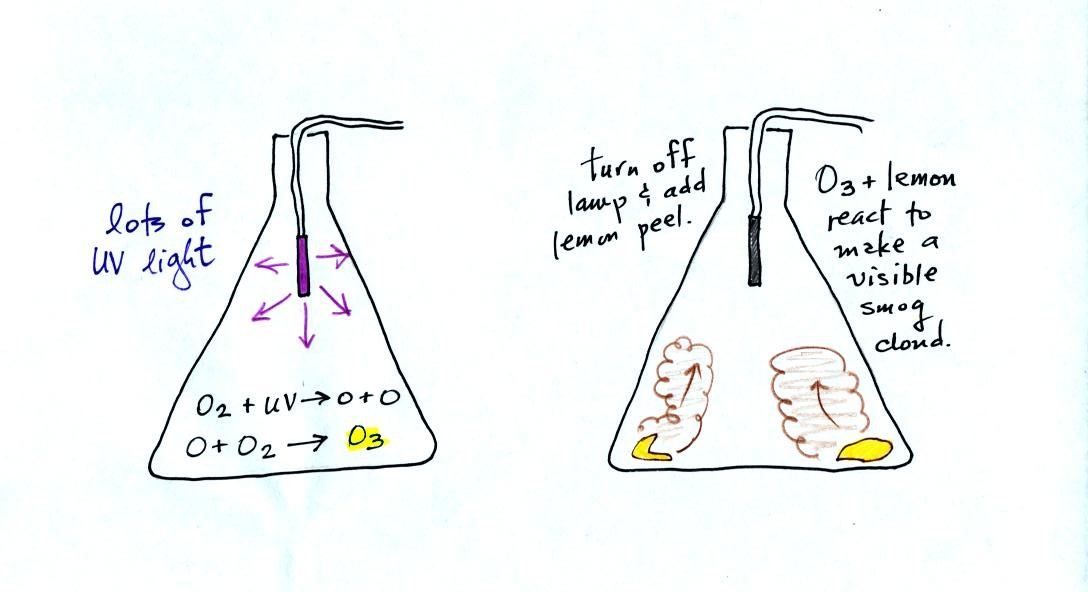

Here's a pictorial summary of the
photochemical smog demonstration.