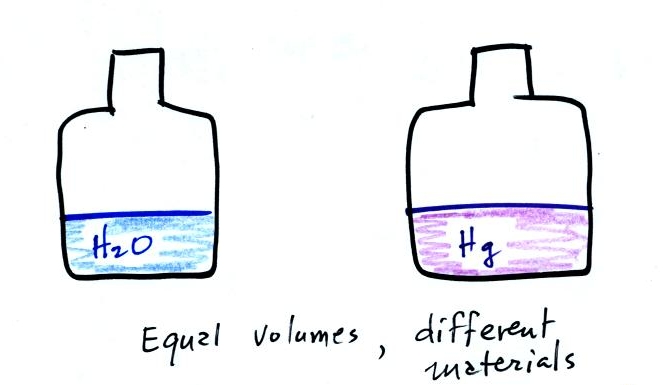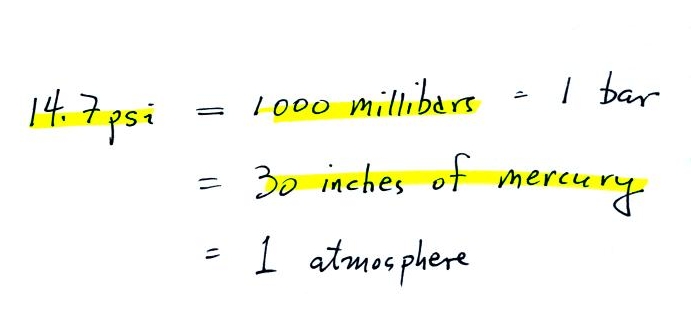Friday Sep. 3, 2010
click here to download today's notes in a
more printer friendly format
The start of a 3-day weekend seemed like a good place for a couple
of songs from the Flobots
("Handlebars" and "By the Time You Get This Message").
All of the names on the Report Signup sheets should now be on the Online Lists. If you name isn't
there and you think it should be, let me know. Sorry about any
misspelled names.
Next Monday is a holiday. There'll be a Practice Quiz review next
Tuesday afternoon from 4-5 pm in Haury 129 (aka Anthropology
129). The Practice Quiz is next Wednesday (it'll start around
2:20 pm).
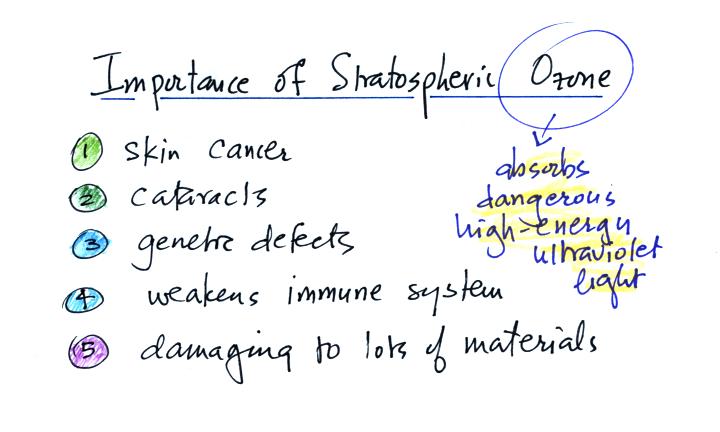
Now that we have finished the section on
air pollutants, here's a list of the key
points for each of the pollutants that we covered.
carbon monoxide
(CO)
colorless, odorless
primary pollutant
incomplete combustion
winter, morning pollutant
temperature inversion layer
|
tropospheric
ozone (O3)
secondary pollutant
summer, afternoon pollutant
Los Angeles - type (photochemical smog)
|
sulfur dioxide
(SO2)
1st pollutant
London - type smog
acid rain
|
particulate
matter (PM)
health hazard
affects visibility |
Before moving into a new and important section on air pressure, we
took a moment to cover a little new material on
stratospheric ozone. Stratospheric ozone (the ozone layer)
absorbs dangerous high-energy ultraviolet light. Earlier this
week we covered tropospheric
ozone which is
a pollutant and a key ingredient in photochemical smog.
This topic is covered on pps. 17-19 in the photocopied ClassNotes.
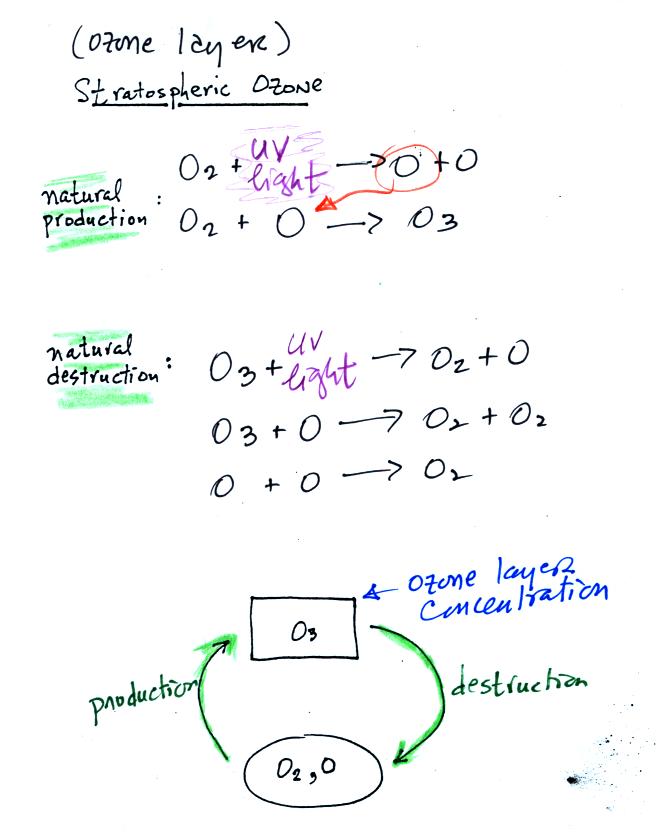
The
top
two
equations
show
how ozone is produced in the stratosphere.
Ultraviolet (UV) light splits an O2 molecule into two O atoms
(photodissociation).
Each of the O atoms can react with O2 to make O3 (ozone).
Ozone is destroyed when it absorbs UV light and is split into O
and O2
(the two pieces move away from each other and don't recombine and
remake ozone). O3 is also destroyed when it reacts with an oxygen
atom (thereby removing one of the "raw ingredients" used to make
ozone). Two molecules of ozone can also react to make 3 molecules
of O2.
The bottom part of the figure attempts to show that the ozone
concentration in the
stratosphere will shift up and down until the natural rates of
production and
destruction
balance each other (analogous to your bank account not changing when
the amount of money deposition and withdrawn are equal). If an
additional man-caused destruction process is added (orange) that will
lower the ozone layer concentration (if someone else starts spending
some of your money, you balance will decrease).
Knowing that you need O2 and UV light to make ozone,
you can
begin
to understand why the ozone layer is found in the middle of the
atmosphere.
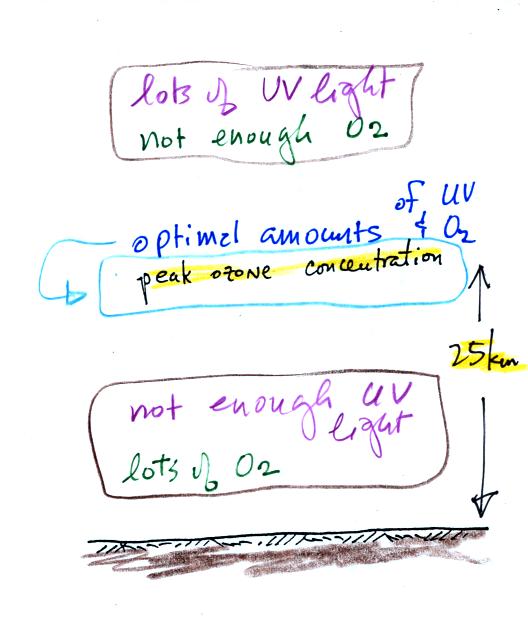
There is plenty of UV light high in
the atmosphere but not much
oxygen (air gets thinner at higher and higher altitude). Near the
ground there is plenty of oxygen but not as much UV light (it is
absorbed by gases above the ground). You find the optimal amounts
of UV light and oxygen somewhere in between, near 25 km altitude.
This next figure lists some of the problems associated
with
exposure to UV light. Thinning of the ozone layer will result in
increased amounts of UV light reaching the ground.
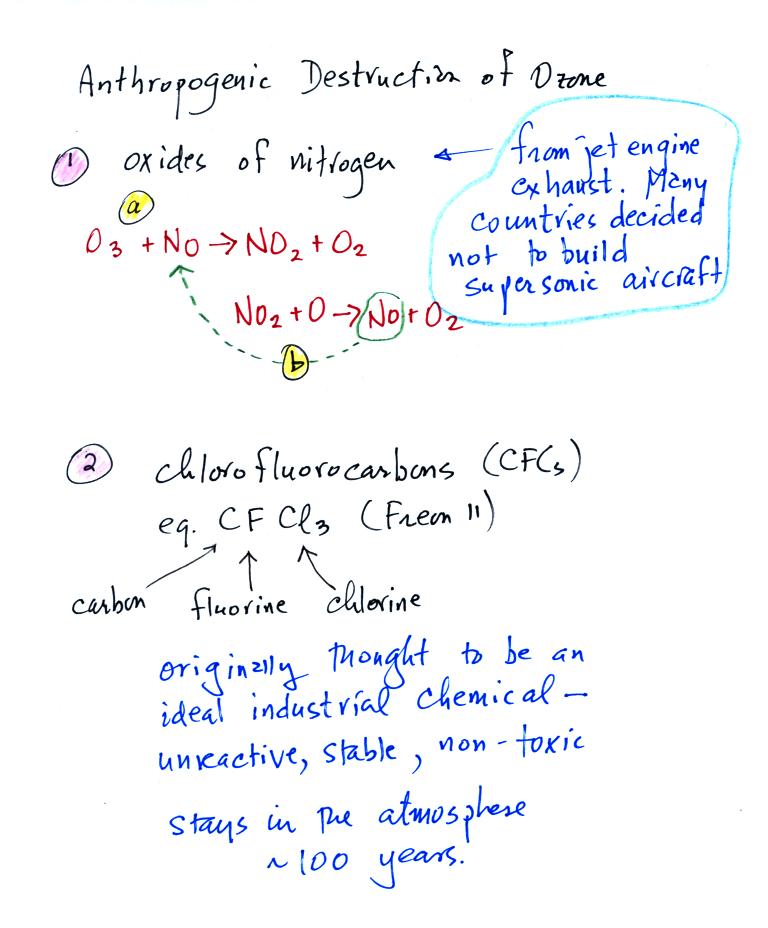
The first set of reactions above involve nitric oxide, NO. First,
NO reacts with O3 to form NO2 and O2
(ordinary molecular oxygen).
Then notice the NO2 reacts with an oxygen atom (which might
otherwise
react with O2 to make O3) to form NO again and O2.
The
NO
is
available
again
to
react
with
and destroy another ozone molecule.
At one time many countries were considering building fleets of
supersonic aircraft that would fly into the stratosphere. The
plans were scrapped partly due to concern that the NO emissions from
these aircraft would damage the ozone layer.
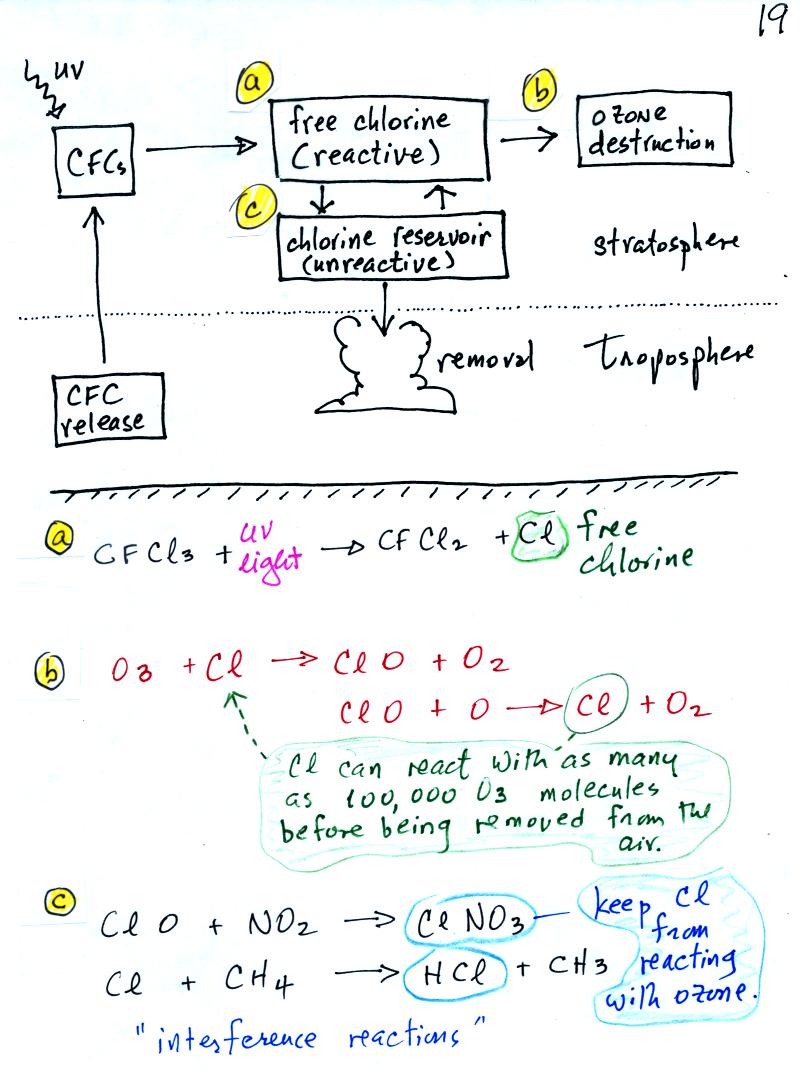
The main threat now comes from chlorofluorocarbons (CFCs). CFCs
were
at
one
time
thought
to
be an ideal industrial
chemical and had a variety of uses.
CFCs are unreactive, non toxic, and stable. Once they get into
the atmosphere they remain there a long time, as much as 100 years.
The
reactions
involving
CFCs
are
shown
on
the next figure.
CFCs released at ground level [lower left corner in the figure
above]
remain in the atmosphere long enough that they can eventually make
their way up into the stratophere. UV light can then break
chlorine
atoms off the CFC molecule [a]. The resulting
"free chlorine" can react with and destroy ozone. This is shown
in (b) above. Note how the chlorine atoms reappears at the end of
the two step reaction. A single chlorine atom can destroy 100,000
ozone molecules.
There are ways of removing chlorine from the atmosphere. A
couple
of these so called "interference reactions" are shown in (c)
above. The reaction products, reservoir molecules
(because they store chlorine), might serve as
condensation nuclei for cloud droplets (the small water drops that
clouds are composed of) or might dissolve in the
water in clouds. In either event the chlorine containing chemical
is removed from the atmosphere by falling precipitation. Clouds
are probably the most effective way of cleaning the atmosphere.
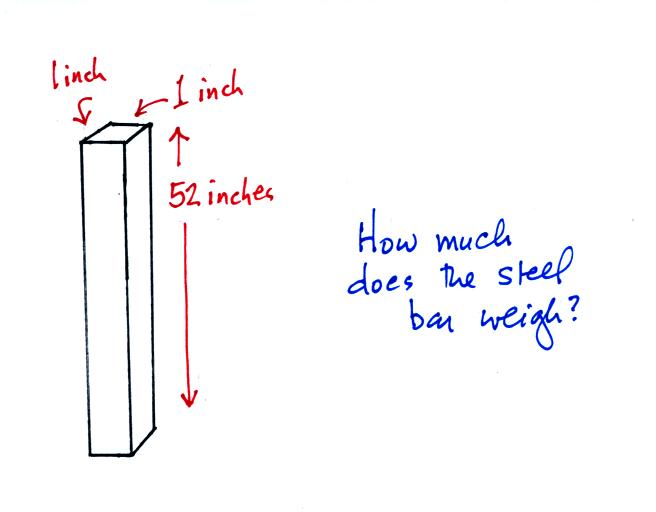
An iron bar was passed around at the
beginning of class. You were supposed to guess how much it
weighed.
We came back to this later in the period.
Bottles containing approximately equal volumes of
water and mercury were passed around in class (thanks for being careful
with the mercury). There is a lot more mass in the bottle of
mercury than in the bottle of water. Because it has more mass the
bottle of mercury also weighs more than the bottle of water (that's
something you can feel). Mercury is much denser than water.
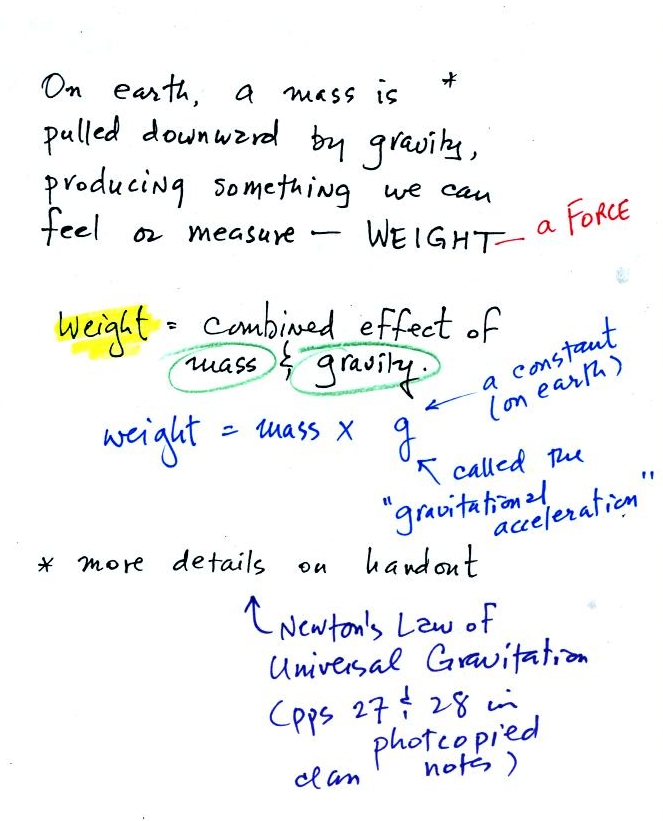
Before we can learn about
atmospheric pressure, we
need to review
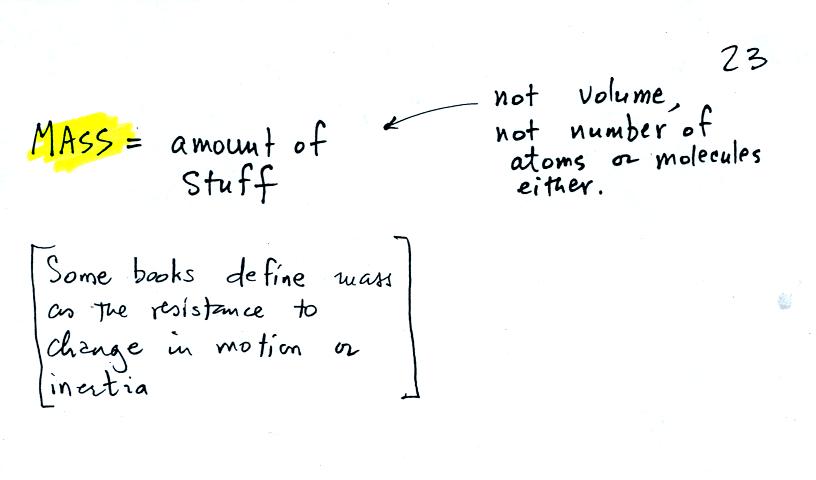
the terms mass and weight. In some textbooks you'll find mass
defined as "amount of stuff" or "amount of a particular
material." Other books will define mass as
inertia or as resistance to change in motion (this comes from Newton's
2nd law of motion, we'll cover that later in the semester). The
next picture
illustrates both these definitions.
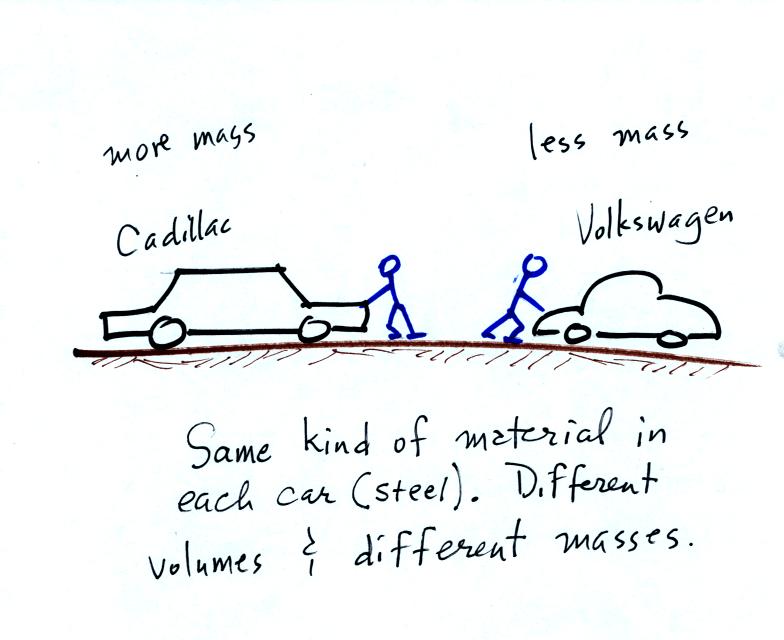
A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down than the
Volkswagen once it is
moving).
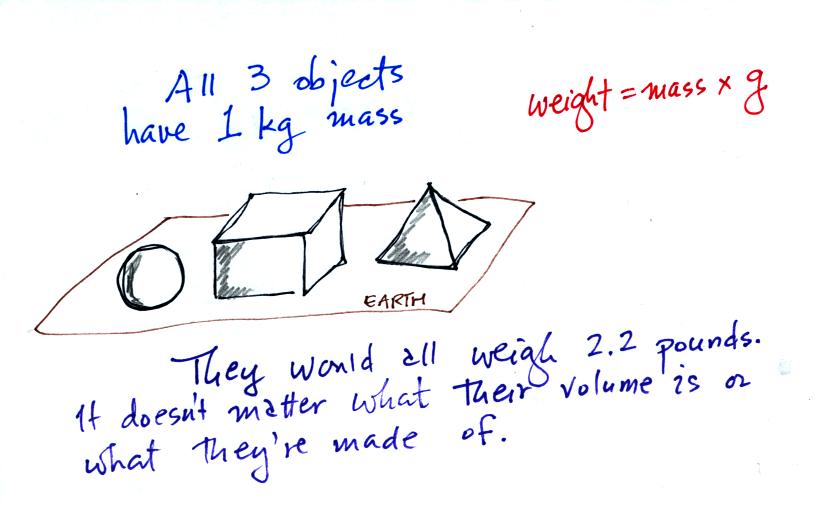
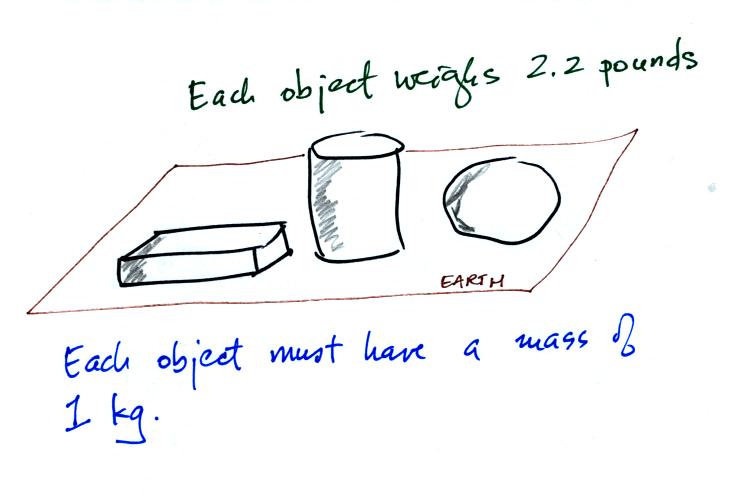
On the earth where the pull of
gravity never changes, any three objects
that all have the same mass
(even if they had different volumes and were made of different
materials) would always have the same weight. Conversely:
When
gravity
is
always
the
same,
three
objects
with the
same weight
would also have the same mass.
The
difference
between
mass
and
weight
is clearer
(perhaps) if you
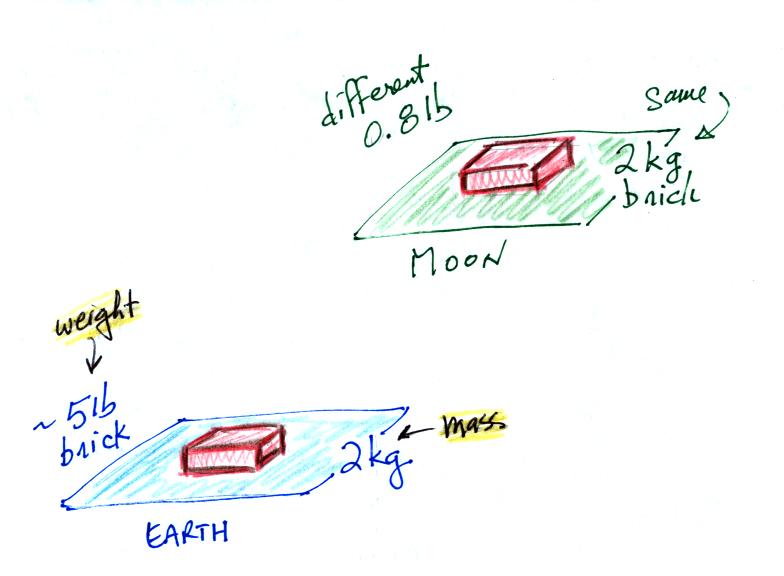
compare the situation on the earth and on the moon.
On the earth a brick with a mass of about 2 kg weighs about 5
pounds. If you carried the brick to the moon it would have the
same mass. But gravity on the moon is weaker than on the
earth. Objects on the moon weigh less than on the earth.
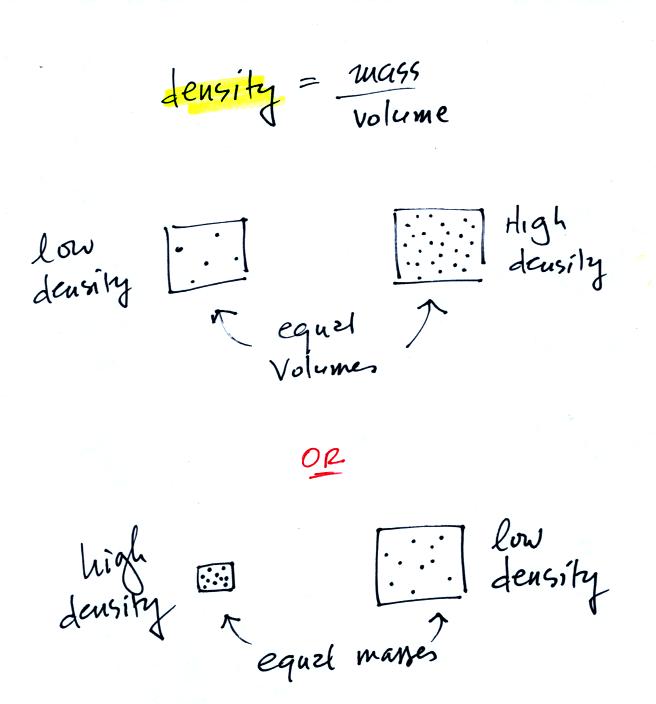
In
the first example there is more mass (more dots) in the right box than
in the left box. Since the two volumes are equal the box at right
has higher density. Equal masses are squeezed into different
volumes in the bottom example. The box with smaller volume has
higher density.
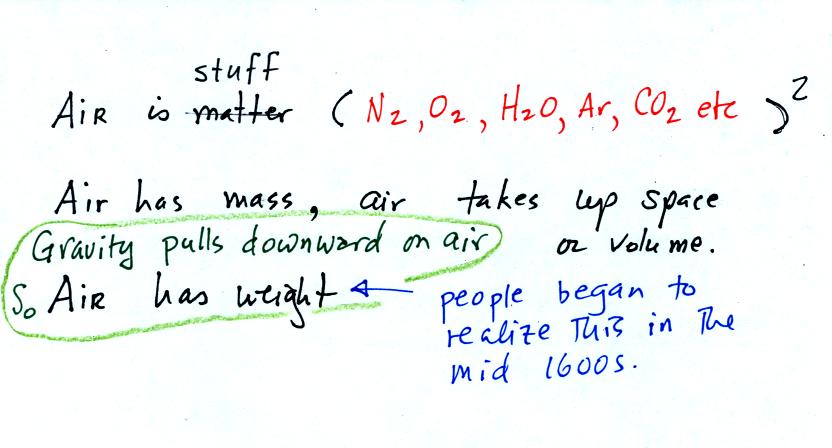
The air
that
surrounds the earth has mass. Gravity pulls downward on the
atmosphere giving it weight. Galileo conducted (in the 1600s) a
simple
experiment
to
prove
that air has weight. The experiment wasn't mentioned
in class.
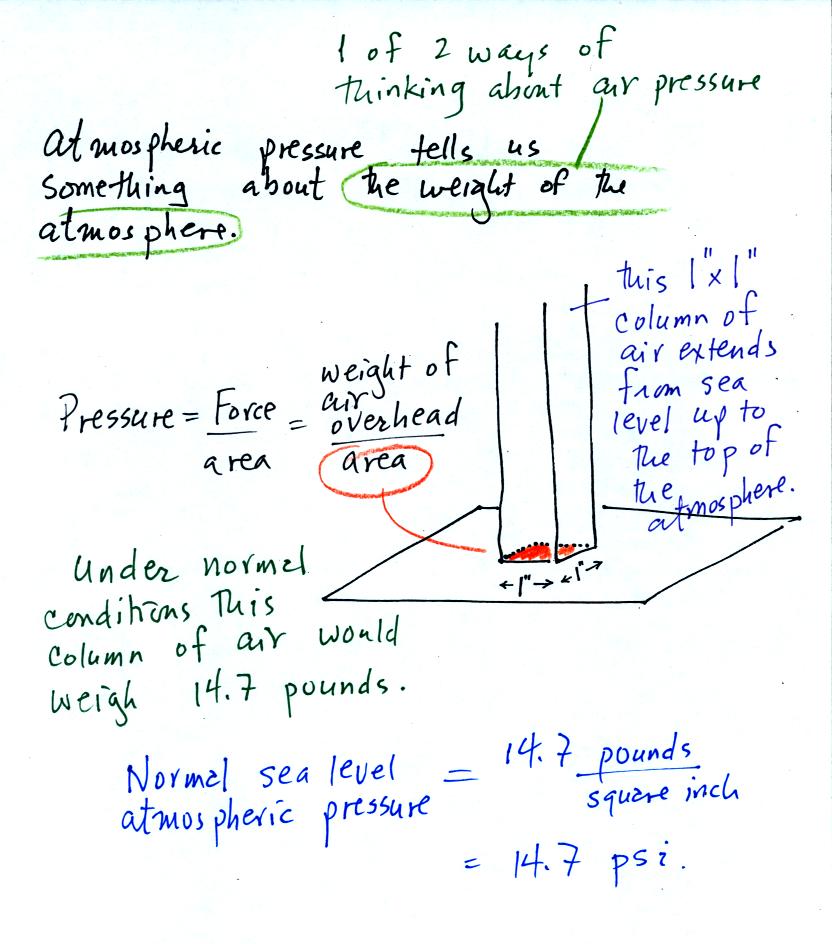
Pressure is defined as force divided by area. Air
pressure is the
weight
of the atmosphere overhead divided by the area the air is resting
on.
Atmospheric pressure is
determined by and tells you something about the weight of the air
overhead. This is one way, a sort of large scale representation,
of understanding air pressure.
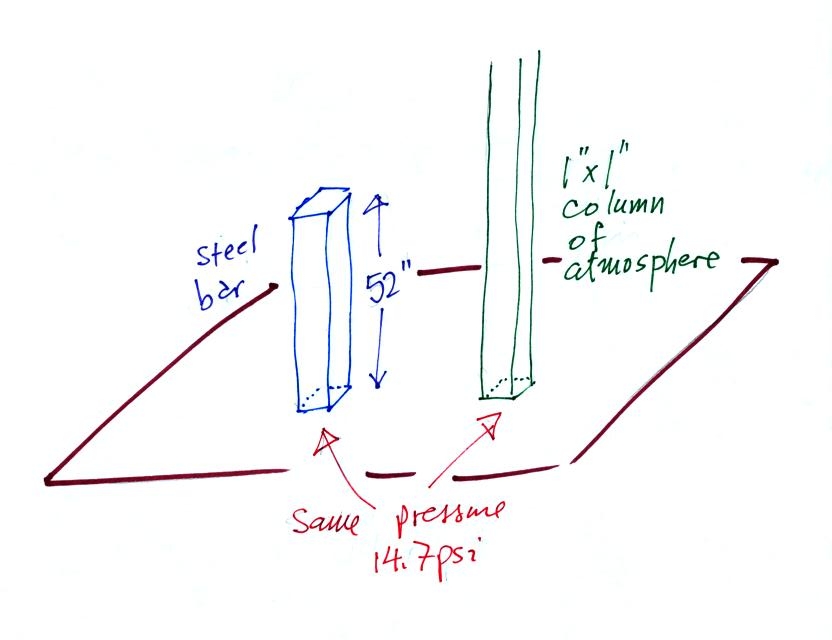
Under normal conditions a 1 inch by 1 inch column of air
stretching
from sea level to the top of the atmosphere will weigh 14.7
pounds. Normal
atmospheric
pressure at sea level
is 14.7 pounds per square inch (psi, the units you use when you fill up
your
car
or
bike
tires
with
air).
Now here's
where the steel bar
comes in. The steel bar also weighs exactly 14.7 pounds (many
people think it is heavier than that). Steel is a lot denser
than air, so a steel bar only needs to be
52 inches tall to have the same weight as an air column that is 100
miles or more tall.
14.7 psi is one weigh of expressing average sea level
pressure. Here are average sea level pressure values in different
units.
Typical sea level
pressure is 14.7 psi or about 1000 millibars
(the
units used by meterologists and the units that we will use in this
class most of the time) or about 30 inches of mercury (refers to
the reading on a mercury barometer). If you ever find
yourself in France needing to fill your
automobile tires with air (I lived in France for a while and owned
a Peugeot
404)
remember that the air compressor scale is
probably calibrated in bars. 2 bars of pressure would be
equivalent to 30 psi.