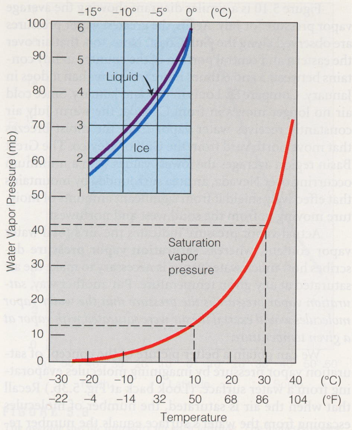
SVP over Liquid and Ice
SVP over ice is less than over water because sublimation
takes more energy than evaporation
If
water surface is not flat, but instead curves like a cloud drop,
then the SVP difference is even larger
So
at equilibrium, more vapor resides over cloud droplets than ice crystals
Ahrens, Meteorology Today 5th Ed.