The energy state of a gas depends on its
Later we will see that these three measurable properties of gases are related to each other by the ideal gas law.
For now we will first describe the macroscopic properties of gases in terms of what is going on at the molecular or microscopic level using the kinetic model. Link to page describing kinetic model.
As we describe how each of these three properties of gases can be understood using the kinetic model, we will examine how each changes in the vertical, i.e., as one moves up and down in Earth's atmosphere. The average vertical structure of temperature, density, and pressure in Earth's atmosphere described below is often called the "static structure" as it does not consider large scale movements of air that we call weather. It is like describing how the average properties of ocean water (e.g., water temperature and salinity) in moving from the ocean bottom to the top of the water without considering ocean currents.
Temperature is determined by the average speed of the molecules making up a substance. The higher the temperature, the faster they move. For gases, this is the random motion of the individual molecules that make up the gas. Random motion is disordered, i.e., individual molecules are equally likely to be moving in any direction. At temperatures common in Earth's atmosphere, the average speed of each molecule is approximately 1000 mi/hr. This is different from what we call "wind" which is ordered movement of air at the macro scale (basically the ordered movement of a fluid in a given direction). For example, when the windspeed is 10 mi/hr, it means that "blobs" of air are moving at 10 mi/hr, but individual molecules are still moving at an average speed of about 1000 mi/hr.
Using the concept of energy, the higher the temperature, the more energy that is possessed by the gas. It should make sense, then, that the higher the temperature, the higher the energy, and the faster the speed at which the molecules are moving.
We sense temperature by touch. Thermorecptor nerve cells in our body are sensitive to the average speed at which air molecules are moving. Similarly, when air molecules strike a thermometer, energy is transferred between the thermometer and the air. The reading on the thermometer is calibrated to read the average thermal motion of all of the air molecules that collide with it.
Often, the Earth's atmosphere is divided into several different layers that are defined according to the typical change in air temperature. (See Image Below).
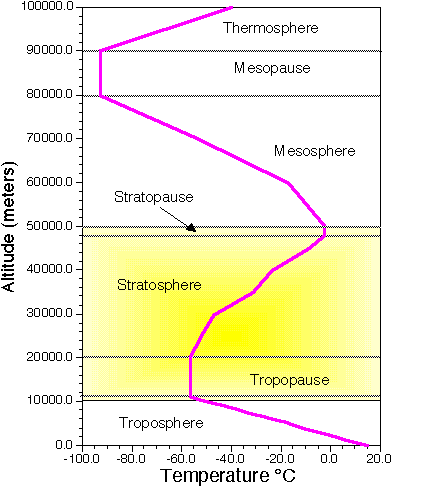 |
| Atmospheric layers defined by structure of air temperature. |
Air density can be defined as the number of air molecules per unit volume. Near sea level there are about 2.7x1019 molecules per cm3(cubic centimeter) or 4.4x1020 molecules per inch3(cubic inch). While this may seem like a lot of molecules in a small area, molecules are very small. By comparison, the number density for solids and liquids is much higher. In a gas there is lots of empty space between the individual molecules.
Air molecules are held near the earth by gravity. In other words, air has weight. Weigh an empty bag, then fill it with air, it now weighs more. In addition gases, like air, are easily compressed, i.e., squeeze a gas together and its number density increases. In other words, we say gases are compressible because they can easily be squeezed into a smaller volume. Solids and liquids on the other hand are not easily compressed.
The weight of all of the air above a given point in the atmosphere squeezes air molecules closer together, which causes their numbers in a given volume to increase (increase in number density). The more air above a level (and hence the more weight of air above a level), the greater the squeezing effect (or compression).
Since air density is the number of air molecules in a given space (volume), air density is typically greatest at the surface or sea level (where it is squeezed by the weight of the entire atmosphere above) and decreases as we move up in the atmosphere because the weight of air above becomes less and hence there is less of a squeezing effect (See Figure Z).
From a microscopic point of view, gas pressure is caused by the collisions of gas molecules on a surface. Each individual collision provides a tiny push (or force) on the surface that it contacts. The sum total of all of these tiny forces determines the air pressure. The physical units for pressure is force per area. More generally, all fluids (liquids and gases) exert pressure on the surfaces of solids that are immersed in them, which is simply the force of the molecules of the fluid bouncing off the solid surface.
The weight of the atmosphere acts as a force upon the underlying surface of the Earth. The amount of force excerted over an area of surface is called atmospheric pressure or air pressure. Near sea level, the average air pressure is about 14.7 pounds per square inch. In this class we will use the unit millibars(mb) to specify air pressure. At sea level the average air pressure is 1013 mb.
Since the air (a gas) is a fluid, the pressure force acts in all directions, not just downward. The pressure force pushing downward due to the weight of the air is the same as the pressure force acting sideways and even upward. If you are having trouble understanding this, make an analogy with another fluid liquid water. Consider a deep swimming pool full of water. The water pressure anywhere in the pool depends on the weight of the water above (that is the deeper you dive downward in the pool, the stronger the water pressure.) The pressure force is not just downward though, it pushes in on your body from all directions. The average air pressure at sea level (1013 mb or sometimes called one atmosphere of pressure) is caused by the weight of all the air above sea level. In the same way water pressure is caused by the weight of water above you. At a depth of 32 feet (9.75 meters) below a water surface, the water pressure is about one atmosphere. Thus, the entire column of air from sea level to outer space weighs as much as a 32 foot column of water. Of course diving deeper into water means you will encouter an increasing water pressure (enough to crush you if you go too deep).
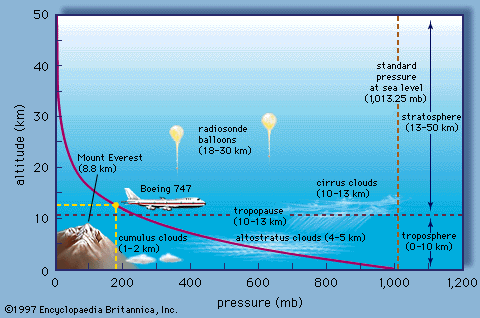 |
| Typical change in air pressure with altitude. Note how rapidly air pressure falls with increasing altitude. |
In the atmosphere, the air pressure at any point depends on the weight per area of the air above that point. As we climb in elevation, fewer air molecules are above us; hence, atmospheric pressure always decreases as you move upward in the atmosphere (See Figure B). Another way to look at it is that the air pressure at any point in the atmosphere is exactly enough to support the weight of the column of air above it. A balance exists between the gravitational force pushing air downward and the upward directed pressure force. This balance is called hydrostatic balance.
In class (Figure C) comparing the rate of pressure changes between water and air.
Air pressure video containing simple experiments demonstrating air pressure.